Abstract
Plant-borne secondary metabolites are attracting high interest for their potential use in agricultural applications, with special reference to the control of arthropod pests. In the present work, the structural elucidation of glycosylated diterpenoid carboxyatractyloside (2) isolated from the roots of Chamaeleon gummifer Cass. (Asteraceae) is reported by means of spectroscopic and spectrometric techniques. Complete identification occurred thanks to one- and two-dimensional NMR experiments, assigning the single protons and carbons, and the stereochemistry by the NOESY correlations. Carboxyatractyloside (2), together with two ent-kaurenes atractyloside (1) and atractyligenin (3), extracted from the roots of C. gummifer, have been tested for their acaricidal and oviposition inhibition activity against the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) Notably, compounds 1–3 were toxic to T. urticae, leading to significant mortality, oviposition inhibition, reduced hatchability of eggs, and natality inhibition. However, at the lowest dose (12.5 µg cm−2) compound 2 was the most effective, leading to mortality > 60% after 5 days exposure, inhibiting oviposition by > 70% and egg hatching by 33%; it also reduced natality by 80%. Overall, these compounds represent valuable candidates to develop novel acaricides for crop protection. Further research on how to develop stable formulations for field use, as well as on non-target effects of these compounds on pollinators and mite biocontrol agents, is ongoing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Atractyloside, carboxyatractyloside, and atractyligenin characterized the C. gummifer extract.
-
Complete structural elucidation of carboxyatractyloside was reported.
-
The toxicity of all diterpenes was tested against the two-spotted spider mite, T. urticae.
-
Carboxyatractyloside exhibited the highest mortality and oviposition inhibition.
Introduction
Nowadays, the growing demographic increase is proportionally linked to the incessant growth in food needs, and to the huge demand for new agricultural spaces (Ray et al. 2013). In recent years, scientific research has aimed at the establishment of new technologies intended at increasing the yield of agricultural crops and quality of products, not forgetting the progressive decrease in land availability, water scarcity, and increasing climatic changes. Scientific development involves advances in the field of phytogenetics, searching for more resistant seeds with a higher yield, in the management of grazing, as well as about the development of biopesticides based on natural products (Pavela and Benelli 2016; Isman 2020).
The latter research field includes a focus on products of natural origin and semi-synthetic derivatives, which can play a fundamental role for managing insects and mites of economic importance (Stevenson et al. 2017; Benelli and Pavela 2018). Indeed, it is estimated that in 2012, natural products and related derivatives accounted for 50% of global sales of agrochemicals (Loso et al. 2017). Therefore, studying the modes of action of secondary metabolites has expanded their use in agricultural applications towards the control of harmful insects and mites (Jankowska et al. 2017). Of note, different researches reported the use of plants as interesting sources of secondary metabolites showing significant toxicity against a growing number of arthropod pests and vectors. Good examples are several plant species belonging to the Apiaceae (Basile et al. 2022; Badalamenti et al. 2021a; Spinozzi et al. 2021), Asparagaceae (Badalamenti et al. 2021b; 2022a), and Asteraceae families (Haris et al. 2022; Mssillou et al. 2022; Kavallieratos et al. 2022), among others (Stevenson et al. 2017; Isman 2020; Pavela et al. 2019a, 2020; Kavallieratos et al. 2021).
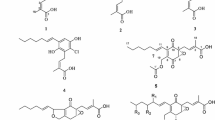
Chamaeleon gummifer Cass., i.e., the scientifically accepted name of the more common Atractylis gummifera L. (Worldfloraonline 2022), belongs to the Asteraceae family. It has a distribution in almost the entire Mediterranean basin (Spain, Portugal, South Italy, Greece, North Morocco, and Algeria). It is a thorny and perennial thistle with wide roots that can reach 40–50 cm, and a diameter of 8–10 cm. The stem, which extends even up to 1 m, is covered with very spiny leaves and, during the full summer days, with intense pink flowers (Vallejo et al. 2009; Bouabid et al. 2019a). This plant is commonly used in regional ethnopharmacology, for example, in Morocco (Kharchoufa et al. 2018); however, it is considered very toxic, and whose mortality depends on the dose used, the age of the subject who uses it, and the vegetal part utilized (roots, leaves, or flowers) (Bouabid et al. 2019a, 2019b). The species is traditionally used against colds, nausea, stomach pain, and dizziness, but also, in the form of an infusion, against blisters or bleeding, as a vermifuge or even as a purgative (Ahid et al. 2012; Hammich et al. 2013). The cases of poisoning due to this plant mention its use for traditional purposes and have taken into consideration the phytochemistry of aqueous or organic extracts, and above all the presence of highly toxic diterpenes such as atractyloside (1), carboxyatractiloside (2), and atractyligenin (3).
The first compound isolated, and then structurally identified, from the roots of C. gummifer Cass. was atractyloside (Lefranc 1868). However, today these diterpenes have also been found in different plants such as Xanthium strumarium L. (Machado et al. 2021), Coffea arabica L. and C. robusta L. Linden (Gao et al. 2021; Hu et al. 2021), and Antennaria rosea subsp. confinis (Greene) R.J.Bayer (Xiao et al. 2019). Atractyloside and carboxitractyloside, extensively investigated for their biological activities (Todisco et al. 2015; Cho et al. 2017), act at the cellular level with inhibition of oxidative phosphorylation in the hepatocytes (Vignais et al. 1978). Essentially, these two secondary metabolites negatively affect the production of ATP in the cell, since, by blocking the transport of adenosine diphosphate (ADP) into the mitochondria, they inhibit the adenine nucleotide translocator (ANT) (Roux et al. 1996; Pebay-Peyroula et al. 2003). The aglycon atractyligenin, due to the absence of isovaleric unit and sugar moiety, resulted significantly less lethal than 1 and 2 (Vignais et al. 1978). Several studies reported different chemical modifications of atractyligenin, supported by excellent biological properties. These experiments included the photoinduced modification of methyl C-20 (Buscemi et al. 2001; 2003), enzymatic transformations of all OH– groups (Monsalve et al. 2005), and the preparation of anti-tumour derivatives (Rosselli et al. 2007; Cotugno et al. 2014). One of these compounds, 15-ketoatractyligenin methyl ester, showed a high activity against several tumour cell lines (Cotugno et al. 2014; Vasaturo et al. 2017; Badalamenti et al. 2022b).
In this work, the first complete structural elucidation of 2 by 1D-NMR (Nuclear Magnetic Resonance) and bidimentional techniques such as 1H-1H-COSY (Correlated Spectroscopy), NOESY (Nuclear Overhauser Effect Spectroscopy), HSQC (Heteronuclear Single Quantum Coherence), and HMBC (Heteronuclear Multiple Bond Correlation) spectroscopy and mass spectrometry (HPLC/ESI/Q-TOF) is presented. Furthermore, compounds 1–3 were evaluated for their acaricidal efficacy and oviposition inhibition activity against the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), a polyphagous mite pest of high agricultural importance (Attia et al. 2013), which has been found resistant to a rather wide number of acaricides exerting their toxicity through different mechanisms of action (van Leuveen et al. 2010; Xu et al. 2018; Wu et al. 2019; Adesanya et al. 2021).
Materials and methods
Plant material
Chamaeleon gummifer roots were collected in Piana degli Albanesi, Sicily (Italy) at the beginning of May 2020. The authentication was carried out by Prof. Vincenzo Ilardi, University of Palermo, Italy. A voucher specimen (PAL MB-2020/84) has been deposited in STEBICEF Department, University of Palermo.
General procedures
Optical rotations, measured in a CH3OH solution, on a JASCO P-1010 digital polarimeter; 1H (Fig. S1) and 13C-NMR spectra (Fig. S2), for compounds 1–2, were recorded at 400/100 MHz in (CH3)2SO (dimethyl sulfoxide, DMSO-d6) unless otherwise noted, on Bruker spectrometers, using the residual solvent signal (δ = 2.50 ppm in 1H and δ = 39.51 ppm in 13C for DMSO) as reference. For the compound 3 proton and carbon spectra were recorded in CD3OD using the residual solvent signal (δ = 3.31 ppm in 1H and δ = 49.15 ppm in 13C for CD3OD) as reference. The sperimental procedures have been previously reported (Badalamenti et al. 2021b).
Extraction and isolation of atractyloside (1), carboxyatractyloside (2), and atractyligenin (3)
The dried roots of C. gummifer (≈ 4.5 kg) were grounded and extracted in CH3OH three times at room temperature (4 L × 3 times). After filtration and evaporation of solvent under reduced pressure, a raw methanolic extract was obtained (98.01 g). This extract, dissolved in distilled water, was partitioned into n-butanol (11.23 g). This latter layer was chromatographed on a silica gel column, eluting with dichloromethane/methanol (99:1 → 90:10 v/v), to give 6 different fractions Cg1-Cg6. The Cg4-Cg5 (4.32 g) fractions were re-chromatographed, using a mixture of dichloromethane/acetone/water (5.5/4/0.5, v/v/v) as eluent, on a silica gel, to give compounds 1 (611 mg) and 2 (453 mg). From the Cg1 fraction (619 mg), after the separation and the purification on silica column, compound 3 (374 mg) was obtained.
Atractyloside (1)
Colorless amorphous solid; [α\({]}_{\mathrm{D}}^{20}=\) − 51.7° (c 0.2, H2O). 1H-NMR (DMSO-d6, 400 MHz) δ 2.17 (1H, m, H-1α), 0.62 (1H, t, J = 12.0 Hz, H-1β), 4.03 (1H, m, H-2α), 2.21 (1H, dd, J = 2.2, 1.7 Hz, H-3β), 1.04 (1H, ddd, 5.4, 5.2, 4.6 Hz, H-3α), 2.60 (1H, bs, H-4), 1.33 (1H, m, H-5β), 1.74 (1H, m, H-6α), 1.50 (1H, m, H-6β), 1.52 (1H, m, H-7β), 1.37 (1H, m, H-7α), 1.00 (1H, d, J = 8.0 Hz, H-9β), 1.52 (1H, m, H-11a), 1.28 (1H, m, H-11b), 1.54 (1H, m, H-12α), 1.39 (1H, m, H-12β), 2.58 (1H, bs, H-13), 1.75 (1H, d, J = 11.0 Hz, H-14α), 1.30 (1H, m, H-14β), 3.61 (1H, m, H-15β), 5.07 (1H, bs, H-17a), 4.97 (1H, bs, H-17b), 0.90 (3H, d, J = 2.6 Hz, CH3-20), 4.55 (1H, d, J = 7.9 Hz, H-1ʹ), 4.66 (1H, t, J = 7.9 Hz, H-2ʹ), 4.32 (1H, t, J = 8.0 Hz, H-3ʹ), 4.00 (1H, t, J = 8.0 Hz, H-4ʹ), 3.43 (1H, m, H-5ʹ), 3.66 (1H, m, H1-6ʹ), 3.60 (1H, m, H2-6ʹ), 2.12 (1H, d, J = 2.30 Hz, H-2ʹʹ), 2.14 (1H, d, J = 2.0 Hz, H-2ʹʹ), 1.96 (1H, sept, J = 6.5 Hz, H-3ʹʹ), 0.87 (6H, d, J = 2.3 Hz, H-4ʹʹ, H-5ʹʹ). 13C-NMR (DMSO-d6, 100 MHz) δ 47.0 (C-1), 72.3 (C-2), 34.1 (C-3), 45.3 (C-4), 48.1 (C-5), 25.0 (C-6), 35.1 (C-7), 47.2 (C-8), 52.5 (C-9), 42.6 (C-10), 17.2 (C-11), 32.0 (C-12), 41.6 (C-13), 35.9 (C-14), 81.0 (C-15), 159.9 (C-16), 107.4 (C-17), 176.0 (C-19), 15.9 (C-20), 98.6 (C-1ʹ), 72.1 (C-2ʹ), 77.0 (C-3ʹ), 73.4 (C-4ʹ), 75.9 (C-5ʹ), 61.8 (C-6ʹ), 171.0 (C-1″), 42.6 (C-2″), 24.5 (C-3″), 22.13 and 22–15 (C-4″-C-5ʹʹʹ). ESIMS (‒) m/z 725.2134 [M‒H]‒ (calcd. for C30H45O16S2, m/z 725.2149). Its physical and spectroscopic data agreed with those reported in the literature (Brucoli et al. 2012).
Carboxyatractyloside (2)
Yellow-white amorphous powder; nauseating smell; [α\({]}_{\mathrm{D}}^{25}=\) − 46.5° (c 0.1, MeOH). For 1H- and 13C-NMR data, see Table 1. ESI–MS ( +) m/z 793.2033 [M + Na]+ (calcd. for 793.2023).
Atractyligenin (3)
White amorphous solid; [α \({]}_{\mathrm{D}}^{25}=\) − 146.3° (c 0.1, EtOH). 1H-NMR (CD3OD-d4, 400 MHz) δ 2.18 (1H, m, H-1α), 0.71 (1H, m, H-1β), 4.17 (1H, m, H-2α), 2.38 (1H, m, H-3β), 1.24 (1H, m, H-3α), 2.63 (1H, bs, H-4), 1.52 (1H, m, H-5β), 1.83 (1H, m, H-6α), 1.68 (1H, m, H-6β), 1.61 (1H, m, H-7β), 1.50 (1H, m, H-7α), 1.03 (1H, d, J = 7.5 Hz, H-9β), 1.70–1.62 (2H, m, H2-11), 1.70–1.62 (2H, m, H2-12), 2.69 (1H, bs, H-13), 1.88 (1H, m, H-14α), 1.40 (1H, dd, J = 4.9, 4.7 Hz, H-14β), 3.80 (1H, s, H-15β), 5.19 (1H, bs, H-17a), 5.06 (1H, bs, H-17b), 1.00 (3H, s, CH3-20). 13C-NMR (CD3OD-d4, 100 MHz) δ 50.3 (C-1), 64.9 (C-2), 38.2 (C-3), 45.0 (C-4), 50.0 (C-5), 26.4 (C-6), 36.1 (C-7), 48.0 (C-8), 54.5 (C-9), 41.6 (C-10), 18.8 (C-11), 33.5 (C-12), 43.7 (C-13), 37.0 (C-14), 83.8 (C-15), 160.2 (C-16), 109.0 (C-17), 179.5 (C-19), 17.2 (C-20). ESIMS ( +) m/z 321.2054 [M + H]+ (calcd. for C19H28O4, m/z 321.2060). Its physical and spectroscopic data agreed with those reported in the literature (Brucoli et al. 2012).
Two-spotted spider mite rearing
Tetranychus urticae adults used in our experiments were obtained from a laboratory mass-rearing at the Crop Research Institute (Prague, Czech Republic). Mites were reared on Phaseolus vulgaris L. plants (> 20 generations) in growth chambers under 25 ± 2 °C and 12:12 h (light:darkness) photoperiod.
Acaricidal experiments
The toxicity of carboxyatractyloside, atractyligenin, and atractyloside, measured as mortality at the 2nd and 5th day of exposure, was determined by tarsal application on T. urticae adults (Pavela 2015). Trials were carried out on blackberry leaf discs, Rubus fruticosus L., sized 1 cm2. Carboxyatractyloside, atractyligenin, and atractyloside were formulated in acetone, and distributed using an automatic pipette. In our trials, 10 µL of each compound diluted in acetone were applied uniformly onto the leaf discs. Tested doses were 12.5, 25, 50 and 100 µg cm−2. After application, the discs were placed in Petri dishes (5 cm) with an agar layer 0.3 cm thick on the bottom. Control discs were treated with acetone. Acetone was let evaporating and then a small brush was employed to move 10 T. urticae females (1–2 days old) on each leaf disc. Petri discs were placed in a growth chamber (16:8 (L:D), 25 °C) and mortality was checked after 2nd and 5th day post-application (Pavela et al. 2017).
A further bioassay was developed to assess the possible inhibitory effect of carboxyatractyloside, atractyligenin, and atractyloside on T. urticae fertility and fecundity. The three above-mentioned molecules were formulated at 12.5, 25, 50 and 100 µg cm−2 and subsenquently applied on the R. fruticosus leaf disks as described above. In each replicate, 10 T. urticae females (1–2 days old) were released on each leaf disc in a Petri dish. All material was moved in a growth chamber [16:8 (L:D), 25 °C] for 24 h. After 24 h, the females were removed from the leaf disks, and the eggs were counted. Eggs were incubated for 5 days at 25 °C, and the number of newly hatched larvae was checked every day (Pavela et al. 2017). Data were presented as: (i) egg hatching (%), (ii) hatching inhibition (%), i.e., how many percent fewer larvae hatched compared to the control; (iii) natality inhibition (%), i.e., by what percentage the F1 population was reduced compared to the control (i.e. reduction of oviposition + reduced hatching). Each experiment was replicated 5 times over different days and with different compound solutions, to account for any variability.
Statistical analysis
In T. urticae experiments, mortality rates post-exposure to carboxyatractyloside, atractyligenin, and atractyloside were corrected through the Abbott (1925) formula, then analyzed by ANOVA followed by Tukey’s HSD test (p < 0.05). Percentages were arcsine square-root transformed before analysis; the software BioStat v5.0 was used for all analyses.
Results and discussions
Chemical analyses
The three ent-kaurene metabolites (1–3) (Fig. 1) were isolated from the methanol extract of C. gummifer roots by different chromatographic columns. Compounds 1 and 3, extensively described chemically and biologically in the literature, presented spectroscopic data in agreement with those by of Brucoli et al. (2012). In this study, the complete spectroscopic and stereochemical investigation of compound 2 was reported for the first time using 1D- and 2D-NMR, polarimetric and mass spectrometry (HPLC–MS) analyses.
Compound 2 was obtained, after several chromatographic columns, as a yellowish powder with an unpleasant odor. The HPLC–MS spectrum showed a molecular ion peak at m/z 793.2033 [M + Na]+ (calcd. for 793.2023), in agreement with a molecular formula of C31H46O18S2Na. The proton and carbon spectrum (Fig. S1 and Fig. S2) and the 1H- and 13C-NMR data (Table 1), showed signals for an ent-kaurenic skeleton, a tetracyclic diterpene, characterized by the presence of a double bond in position C-16/C-17 [δC = 159.44 (C-16) and δC = 107.54 (C-17)], two carbonyl functions (C-18 and C-19), with the same carbonic value of chemical shift (δC = 170.90 ppm), linked in position 4, an axial hydroxyl group in position 15 [δH = 3.59 (H-15)] and, finally, an angular methyl (C-20), bonded to carbon C-10, at δC = 16.38 ppm (δH = 0.94, s, 3H, H-20). Furthermore, signals for a glucoside derivative were observed. In fact, using the COSY, HSQC, and HMBC couplings, the entire structure of carboxyatractyloside (2) was determined. The correlation spot presented in the HMBC spectrum, between the anomeric proton H1ʹ (δH = 4.54 ppm, d) and the aglycone C-2 (δC = 73.42 ppm), clearly indicated the exact link between the sugar moiety and the terpenoid skeleton (Fig. 2). Finally, characteristic signals of isovaleric acid, a branched-chain saturated fatty acid, bound to C-2ʹ of the glycosidic unit (correlation spot, in the HMBC spectrum, between proton H-2ʹ and C-1ʹʹ) were found to be quite distinguishable in the proton spectrum: two terminal methyls, doublets, at δH 0.86–0.88 ppm and the unmistakable nonet for H-3 (δH = 1.95 ppm). For the exact stereochemistry, the NOESY correlation between the H-2α with the methyl protons (3H-20α) confirmed the β-glycosidic bond between the sugar portion and the aglycone (Fig. 2).
Acaricidal activity against T. urticae mites
Considering the key agricultural importance of polyphagous T. urticae populations (Wybouw et al. 2018), and their fast-growing resistance to several currently employed acaricides (Wu et al. 2019; Adesanya et al. 2021; Alsay and Ay 2022), the development of novel and reliable products in the Integrated Pest Management (IPM) scenario is a field of research interest. A growing number of botanical-based formulations are being considered for T. urticae management (Pavela et al. 2017, 2018; Benelli et al. 2017). Herein, all the three tested substances, i.e., carboxyatractyloside, atractyligenin, and atractyloside, showed significant toxicity against T. urticae (Tables 2, 3 and 4), in terms of mite mortality, inhibition of oviposition, lower hatchability of eggs, and overall inhibition of natality. However, differences in efficacy between the three molecules were found. Comparing the lowest tested dose, i.e., 12.5 µg cm−2, carboxyatractyloside (2) appears to be the most effective compound, leading to mortality higher than 63% on the 5th day post-application, inhibiting oviposition by more than 70%, and inhibiting the egg hatching by 33.3%. Overall, natality was reduced by 80.1% (Table 2). A significant efficiency was also found for atractyligenin, where testing 12.5 µg cm−2 led to mortality of 50.5% on the 5th day post-application and a total natality of 73.2% (Table 3). The least effective substance was atractyloside, which, when tested at 12.5 µg cm−2 achieved only 34.6% mortality and natality reduction of 45.2% (Table 4).
As outlined above, T. urticae is considered one of the most dangerous crop pests, characterized by the rapid development of acaricide-resistant populations (Raworth 2001). It is therefore very important to focus on new acaricidal substances with novel modes of action, which could become a suitable alternative to existing active ingredients. In the current study, we tested natural ent-kaurene diterpenoids isolated from C. gummifer against T. urticae. Of the substances that we evaluated through acaricidal tests, carboxyatractyloside and atractyligenin showed promising acaricidal efficiency, when at the lowest dose we tested, 12.5 µg cm−2, more than 50% mortality of adults was detected on the 5th day after application, and for carboxyatractyloside, at the same time it was detected greater than 80% inhibition of natality. The dose tested here, i.e., 12.5 µg cm−2, corresponds approximately to an application concentration of 0.125%. In this scenario, about 0.2% could be considered as a lethal concentration, then the tested substances carboxyatractyloside and atractyligenin are more effective than some essential oils. For example, Afify et al. (2012) tested EOs from Matricaria chamomilla L., Origanum majorana L. and Eucalyptus sp. against T. urticae, for which the LC50 were found to be 0.65, 1.84, and 2.18%, respectively, and for eggs 1.17, 6.26, and 7.33%, respectively. The LD50 of these compounds were also lower than that found for the root extracts of Saponaria officinalis L., for which an LC50 of 1.18% was estimated, based on which new botanical acaricides were developed (Pavela 2017).
On the other hand, the efficacy reported in our study was lower than that of Onosma visianii Clem. root extract, showing a LD50 of 2.6 µg cm−2 at the 5th day post-application (Sut et al. 2017), as well as than the Drimia pancration (Steinh.) J.C.Manning & Goldblatt extract, for which the LD50 was 8.5 µg cm−2 (Badalamenti et al. 2022a, b).
The substances tested here show a promising effect on the mortality and fertility reduction of T. urticae, which deepens with time after application. Extracts or compounds that could reduce the fertility of T. urticae are important since this pest multiplies and matures quickly. Therefore, it is necessary to reduce its population density as quickly as possible to minimize the damage caused by the sucking of these phytophagous mites, as well as their vector activity of numerous plant pathogens. Further tests will be required regarding the effectiveness of the compounds proposed in this study on non-target organisms to estimate their environmental safety, as well as the persistence of the effect or the possibility of a synergistic increase in the effectiveness of the substances carboxyatractyloside and atractyligenin.
Conclusions
In this study on the root extract of C. gummifer by mean of 1D- and 2D-NMR, NOESY, and MS spectra, the full stereochemical structure of carboxyatractyloside (2) was revealed for the first time. This diterpene, together with the other two compounds, atractyloside and atractyligenin (1–3), were evaluated for their potential activity as an acaricide against T. urticae. Overall, compounds 1–3, with special reference to carboxyatractyloside, represent valuable candidates to develop novel acaricides for crop protection. However, additional research efforts are still needed to include them in highly stable formulations, such as nanoemulsions for field use (Pavoni et al. 2019; Pavela et al. 2019b). Further research on the possible non-target effects of these compounds on pollinators and mite biocontrol agents is ongoing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. https://doi.org/10.1093/jee/18.2.265a
Adesanya AW, Lavine MD, Moural TW, Lavine LC, Zhu F, Walsh DB (2021) Mechanisms and management of acaricide resistance for Tetranychus urticae in agroecosystems. J Pest Sci 94:639–663. https://doi.org/10.1007/s10340-021-01342-x
Afify AEMR, Ali FS, Turky AF (2012) Control of Tetranychus urticae Koch by extracts of three essential oils of chamomile, marjoram and Eucalyptus. Asian Pac J Trop Biomed 2:24–30. https://doi.org/10.1016/S2221-1691(11)60184-6
Ahid S, Ait el Cadi M, Meddah B, Cherah Y (2012) Atractylis gummifera : de l’intoxication aux méthodes analytiques. Ann Biol Clin 70:263–268. https://doi.org/10.1684/abc.2012.0699
Alsay S, Ay R (2022) Development of resistance to a mixture of spiromesifen and abamectin and cross resistance in Tetranychus urticae. Syst Appl Acarol 27:1857–1866. https://doi.org/10.11158/saa.27.10.1
Attia S, Grissa KL, Lognay G, Bitume E, Hance T, Mailleux AC (2013) A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J Pest Sci 86:361–386. https://doi.org/10.1007/s10340-013-0503-0
Badalamenti N, Ilardi V, Bruno M, Pavela R, Boukouvala MC, Kavallieratos NG, Maggi F, Canale A, Benelli G (2021a) Chemical composition and broad-spectrum insecticidal activity of the flower essential oil from an ancient Sicilian food plant. Ridolfia Segetum Agricu 11:304. https://doi.org/10.3390/agriculture11040304
Badalamenti N, Rosselli S, Zito P, Bruno M (2021b) Phytochemical profile and insecticidal activity of Drimia pancration (Asparagaceae) against adults of Stegobium paniceum (Anobiidae). Nat Prod Res 35:4468–4478. https://doi.org/10.1080/14786419.2020.1729154
Badalamenti N, Bruno M, Pavela R, Maggi F, Marinelli O, Zeppa L, Benelli G, Canale A (2022a) Acaricidal activity of bufadienolides isolated from Drimia pancration against Tetranychus urticae, and structural elucidation of arenobufagin-3-O-α-L-rhamnopyranoside. Plants 11:1629. https://doi.org/10.3390/plants11131629
Badalamenti N, Vaglica A, Maggio A, Bruno M, Quassinti L, Bramucci M, Maggi F (2022b) Cytotoxic activity of several ent-kaurane derivatives of atractyligenin. Synthesis of unreported diterpenic skeleton by chemical rearrangement. Phytochemistry 204:113435. https://doi.org/10.1016/j.phytochem.2022.113435
Basile S, Badalamenti N, Riccobono O, Guarino S, Ilardi V, Bruno M, Peri E (2022) Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 27:588. https://doi.org/10.3390/molecules27030588
Benelli G, Pavela R (2018) Beyond mosquitoes—Essential oil toxicity and repellency against bloodsucking insects. Ind Crop Prod 117:382–392. https://doi.org/10.1016/j.indcrop.2018.02.072
Benelli G, Pavela R, Canale A, Nicoletti M, Petrelli R, Cappellacci L, Galassi R, Maggi F (2017) Isofuranodiene and germacrone from Smyrnium olusatrum essential oil as acaricides and oviposition inhibitors against Tetranychus urticae: impact of chemical stabilization of isofuranodiene by interaction with silver triflate. J Pest Sci 90:693–699. https://doi.org/10.1007/s10340-016-0829-5
Bouabid K, Lamchouri F, Toufik H, Faouzi MEA (2019a) Inventory of poisonings and toxicological studies carried out on Atractylis gummifera L.: a review. Plant Sci Today 6:457–464. https://doi.org/10.14719/pst.2019.6.4.582
Bouabid K, Lamchouri F, Toufik H, Boulfia M, Senhaji S, Faouzi MEA (2019b) In vivo anti-diabetic effect of aqueous and methanolic macerated extracts of Atractylis gummifera. Bangladesh J Pharmacol 14:67–73. https://doi.org/10.3329/bjp.v14i2.38870
Brucoli F, Borrello MT, Stapleton P, Parkinson GN, Gibbons S (2012) Structural characterization and antimicrobial evaluation of atractyloside, atractyligenin, and 15-didehydroatractyligenin methyl ester. J Nat Prod 75:1070–1075. https://doi.org/10.1021/np300080w
Buscemi S, Rosselli S, Bruno M, Vivona N, Piozzi F (2001) Photoinduced functionalization of C-20 methyl group in nor-ditrpene atractyligenin. Tetrahedron Lett 42:8289–8291. https://doi.org/10.1016/S0040-4039(01)01772-5
Buscemi S, Rosselli S, Bruno M, Vivona N, Piozzi F (2003) Photoinduced functionalization of diterpenes. Transformation of the C-20 methyl of atractyligenin into a methylene-carbomethoxy or methylene-carboxyamide group. J Photochem Photobiol Chem 155:145–149. https://doi.org/10.1016/S1010-6030(02)00373-8
Cho J, Zhang Y, Park SY, Joseph AM, Han C, Park HJ, Kalavalapalli S, Chun SK, Morgan D, Kim JS, Someya S, Mathews CE, Lee YJ, Wohlgemuth SE, Sunny NE, Lee HY, Choi Cheol S, Shiratsuchi T, Oh SP, Terada N (2017) Mitochondrial ATP transporter depletion protects mice against liver steatosis and insulin resistance. Nature Commun 8:14477. https://doi.org/10.1038/ncomms14477
Cotugno R, Gallotta D, Dal Piaz F, Apicella I, De Falco S, Rosselli S, Bruno M, Belisario MA (2014) Powerful tumor cell growth-inhibiting activity of a synthetic derivative of atractyligenin: Involvement of PI3K/Akt pathway and thioredoxin system. Biochim Biophys Acta 1840:1135–1144. https://doi.org/10.1016/j.bbagen.2013.11.023
Gao C, Tello E, Peterson DG (2021) Identification of coffee compounds that suppress bitterness of brew. Food Chem 350:129225. https://doi.org/10.1016/j.foodchem.2021.129225
Hammich V, Merad R, Azzouz M (2013) Plantes toxiques à usage médicinal du pourtour méditerranéen. Springer, Paris, p 63
Haris A, Azeem M, Binyameen M (2022) Mosquito repellent potential of Carpesium abrotanoides essential oil and its main components against a dengue vector, Aedes aegypti (Diptera: Culicidae). J Med Entomol 59:801–809. https://doi.org/10.1093/jme/tjac009
Hu G, Peng X, Dong D, Nian Y, Gao Y, Wang X, Hong D, Qiu M (2021) New ent-kaurane diterpenes from the roasted arabica coffee beans and molecular docking to α-glucosidase. Food Chem 345:128823. https://doi.org/10.1016/j.foodchem.2020.128823
Isman MB (2020) Botanical insecticides in the twenty-first century—fulfilling their promise? Annual Rev Entomol 65:233–249. https://doi.org/10.1146/annurev-ento-011019-025010
Jankowska M, Rogalska J, Wyszkowska J, Stankiewicz M (2017) Molecular targets for components of essential oils in the insect nervous system—a review. Molecules 23:34. https://doi.org/10.3390/molecules23010034
Kavallieratos NG, Boukouvala MC, Ntalaka CF, Skourti A, Nika EP, Maggi F, Spinozzi E, Mazzara E, Petrelli R, Lupidi G, Giordani C, Benelli G (2021) Efficacy of 12 commercial essential oils as wheat protectants against stored-product beetles, and their acetylcholinesterase inhibitory activity. Entomol Gen 41:385–414. https://doi.org/10.1127/entomologia/2021/1255
Kavallieratos NG, Nika EP, Skourti A, Boukouvala MC, Ntalaka CT, Maggi F, Spinozzi E, Petrelli R, Perinelli DR, Benelli G, Canale A, Bonacucina G (2022) Carlina acaulis essential oil nanoemulsion as a new grain protectant against different developmental stages of three stored-product beetles. Pest Manag Sci 78:2434–2442. https://doi.org/10.1002/ps.6877
Kharchoufa L, Merrouni IA, Yamani A, Elachouri M (2018) Profile on medicinal plants used by the people of North Eastern Morocco: toxicity concerns. Toxicon 154:90–113. https://doi.org/10.1016/j.toxicon.2018.09.003
Lefranc E (1868) Sur l’acide atractylique et les atractylates, produits immédiats de la racine de l’Atractylis gummifera. Comptes Rendus 69:954–961
Loso MR, Garizi N, Hegde VB, Hunter JE, Sparks TC (2017) Lead generation in crop protection research: a portfolio approach to agrochemical discovery. Pest Manag Sci 73:678–685. https://doi.org/10.1002/ps.4336
Machado M, Queiroz CRR, Wilson TM, Sousa DER, Castro MB, Saravia A, Lee ST, Armien AG, Barros SS, Riet-Correa F (2021) Endemic Xanthium strumarium poisoning in cattle in flooded areas of the Araguari River, Minas Gerais, Brazil. Toxicon 200:23–29. https://doi.org/10.1016/j.toxicon.2021.06.019
Monsalve LN, Rosselli S, Bruno M, Baldessari A (2005) Enzime-catalyzes transformations of ent-kaurane diterpenoids. Eur J Org Chem 10:2106–2115. https://doi.org/10.1002/ejoc.200400862
Mssillou I, Agour A, Allali A, Saghrouchni H, Bourhia M, El Moussaoui A, Salamatullah AM, Alzahrani A, Aboul-Soud MAM, Giesy JP, Lyoussi B, Derwich E (2022) Antioxidant, antimicrobial, and insecticidal properties of a chemically characterized essential oil from the leaves of Dittrichia viscosa L. Molecules 27:2282. https://doi.org/10.3390/molecules27072282
Pavela R (2015) Acaricidal properties of extracts and major furanochromenes from the seeds of Ammi visnaga Linn. against Tetranychus urticae Koch. Ind Crops Prod 67:108–113. https://doi.org/10.1016/j.indcrop.2015.01.011
Pavela R (2017) Extract from the roots of Saponaria officinalis as a potential acaricide against Tetranychus urticae. J Pest Sci 90:683–692. https://doi.org/10.1007/s10340-016-0828-6
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci 21:1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005
Pavela R, Murugan K, Canale A, Benelli G (2017) Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Ind Crops Prod 97:338–344. https://doi.org/10.1016/j.indcrop.2016.12.046
Pavela R, Dall’Acqua S, Sut S, Baldan V, Kamte SLN, Nya PCB, Cappellacci L, Petrelli R, Nicoletti M, Canale A, Maggi F, Benelli G (2018) Oviposition inhibitory activity of the Mexican sunflower Tithonia diversifolia (Asteraceae) polar extracts against the two-spotted spider mite Tetranychus urticae (Tetranychidae). Physiol Mol Plant Pathol 101:85–92. https://doi.org/10.1016/j.pmpp.2016.11.002
Pavela R, Maggi F, Iannarelli R, Benelli G (2019a) Plant extracts for developing mosquito larvicides: from laboratory to the field, with insights on the modes of action. Acta Trop 193:236–271. https://doi.org/10.1016/j.actatropica.2019.01.019
Pavela R, Pavoni L, Bonacucina G, Cespi M, Kavallieratos NG, Cappellacci L, Petrelli R, Maggi F, Benelli G (2019b) Rationale for developing novel mosquito larvicides based on isofuranodiene microemulsions. J Pest Sci 92:909–921. https://doi.org/10.1007/s10340-018-01076-3
Pavela R, Morshedloo MR, Mumivand H, Khorsand GJ, Karami A, Maggi F, Desneux N, Benelli G (2020) Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: insecticidal activity and safety evaluation on non-target earthworms. Entomol Gen 40:421–435. https://doi.org/10.1127/entomologia/2020/1131
Pavoni L, Pavela R, Cespi M, Bonacucina G, Maggi F, Zeni V, Canale A, Lucchi A, Bruschi F, Benelli G (2019) Green micro-and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 9:1285. https://doi.org/10.3390/nano9091285
Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJM, Brandolin G (2003) Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426:39–44. https://doi.org/10.1038/nature02056
Raworth DA (2001) Control of two-spotted spider mite by Phytoseiulus persimilis. J Asia-Pac Entomol 7:5–11. https://doi.org/10.1016/S1226-8615(08)60117-X
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8:e66428. https://doi.org/10.1371/journal.pone.0066428
Rosselli S, Bruno M, Maggio A, Bellone G, Chen TH, Bastow KF, Lee KH (2007) Cytotoxic activity of some natural and synthetic ent-kauranes. J Nat Prod 70:347–352. https://doi.org/10.1021/np060504w
Roux P, Le Saux A, Fiore C, Schwimmer C, Dianoux AC, Trezeguet V, Vignais PV, Lauquin GJ, Brandolin G (1996) Fluorometric titration of the mitochondrial ADP/ATP carrier protein in muscle homogenate with atractyloside derivatives. Anal Biochem 234:31–37. https://doi.org/10.1006/abio.1996.0046
Spinozzi E, Maggi F, Bonacucina G, Pavela R, Boukouvala MC, Kavallieratos NG, Canale A, Romano D, Desnuex N, Wilke ABB, Beier JC, Benelli G (2021) Apiaceae essential oils and their constituents as insecticides against mosquitoes-a review. Ind Crops Prod 171:113892. https://doi.org/10.1016/j.indcrop.2021.113892
Stevenson PC, Isman MB, Belmain SR (2017) Pesticidal plants in Africa: a global vision of new biological control products from local uses. Ind Crops Prod 110:2–9. https://doi.org/10.1016/j.indcrop.2017.08.034
Sut S, Pavela R, Kolarčik V, Cappellacci L, Petrelli R, Maggi F, Dall’Acqua S, Benelli G, (2017) Identification of Onosma visianii roots extract and purified shikonin derivatives as potential acaricidal agents against Tetranychus urticae. Molecules 22:1002. https://doi.org/10.3390/molecules22061002
Todisco S, Di Noia MA, Onofrio A, Parisi G, Punzi G, Redavid G, De Grassi A, Pierri CL (2015) Identification of new highly selective inhibitors of the human ADP/ATP carriers by molecular docking and in vitro transport assays. Biochem Pharmacol 100:112–132. https://doi.org/10.1016/j.bcp.2015.11.019
Vallejo JR, Peral D, Gemio P, Carrasco M, Heinrich C (2009) Atractylis gummifera and Centaurea ornata in the Province of Badajoz (Extremadura, Spain)-Ethnopharmacological importance and toxicological risk. J Ethnopharmacol 126:366–370. https://doi.org/10.1016/j.jep.2009.08.036
van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572. https://doi.org/10.1016/j.ibmb.2010.05.008
Vasaturo M, Fiengo L, De Tommasi N, Sabatino L, Ziccardi P, Colantuoni V, Bruno M, Cerchia C, Novellino E, Lupo A, Lavecchia A, Dal Piaz F (2017) A compound-based proteomic approach discloses 15-ketoatractyligenin methyl ester as a new PPARγ partial agonist with anti-proliferative ability. Sci Rep 7:41273. https://doi.org/10.1038/srep41273
Vignais PM, Vignais PV, Defaye G (1978) In Atractyloside: Chemistry, Biochemistry and Toxicology. Piccin Medical Books, Padova, Italy, pp 39–68
Worldfloraonline. http://www.worldfloraonline.org/taxon/wfo-0000129147. Accessed on: 12 Jul 2022
Wu M, Adesanya AW, Morales MA, Walsh DB, Lavine LC, Lavine MD, Zhu F (2019) Multiple acaricide resistance and underlying mechanisms in Tetranychus urticae on hops. J Pest Sci 92:543–555. https://doi.org/10.1007/s10340-018-1050-5
Wybouw N, Van Leeuwen T, Dermauw W (2018) A massive incorporation of microbial genes into the genome of Tetranychus urticae, a polyphagous arthropod herbivore. Insect Mol Biol 27:333–351. https://doi.org/10.1111/imb.12374
Xiao Y, Lv L, Gou P, Xie H (2019) Acyl atractyligenin and carboxyatractyligenin glycosides from Antennaria rosea subsp. confinis. Phytochemistry 157:151–157. https://doi.org/10.1016/j.phytochem.2018.10.018
Xu D, He Y, Zhang Y, Xie W, Wu Q, Wang S (2018) Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pest Biochem Physiol 150:89–96. https://doi.org/10.1016/j.pestbp.2018.07.008
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. The assistance of the staff is gratefully appreciated. This work was supported by grant from MIUR-ITALY PRIN 2017 (Project N. 2017A95NCJ). R. Pavela would like to thank the Ministry of Agriculture of the Czech Republic for its financial support concerning botanical pesticide research (Project QK1910072).
Author information
Authors and Affiliations
Contributions
MB, RP, FM, and GB conceived the original project. NB and RP performed experiments. RP and GB carried out statistical analyses. NB, MB, RP, FM, and GB wrote the first draft of the manuscript. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Additional information
Communicated by Nicolas Desneux.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badalamenti, N., Bruno, M., Pavela, R. et al. Structural characterization of carboxyatractyloside and acaricidal activity of natural ent-kaurene diterpenoids isolated from Chamaeleon gummifer against Tetranychus urticae. J Pest Sci 97, 911–920 (2024). https://doi.org/10.1007/s10340-023-01679-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01679-5