Abstract
The outbreak of the fall armyworm, Spodoptera frugiperda, in Africa has led to several recommendations of insecticides, including biopesticides. However, the effects of these products on the environment, especially on parasitoids have not been assessed under field conditions. Here, we investigated the effect of commonly used biopesticides on S. frugiperda management and larval parasitoids of S. frugiperda in northern Ghana. The experiments were conducted both on-station in Wa and Nyankpala and on-farm in Wa during the 2020 rainy season. Active ingredients tested included neem oil (3% Azadirachtin), maltodextrin (282 g/l), 55% Bacillus thuringiensis (Bt) combined with 45% Monosultap, and a Pieris rapae granulosis virus combined with 5% Bt. A chemical insecticide based on emamectin benzoate and acetamiprid was used as positive control while non-treated maize plots were considered as untreated control. The two most abundant parasitoids in Wa were Coccygidium luteum and Chelonus bifoveolatus, while in Nyankpala they were C. luteum and Meteorus sp. Total larval parasitism rates on-station were 18.7% and 17.6% in Wa and Nyankpala, respectively, and 8.8% in Wa on-farm. Parasitoid species diversity and evenness indexes did not vary among treatments, but parasitism rates were significantly lower with the chemical on-station in Wa and with the virus and Bt product in Nyankpala. Untreated maize plots showed the highest larval density and plant damage, the highest cob damage, and generated the lowest yields. The other treatments showed hardly any difference in cob damage and yields, suggesting that biopesticides should be preferred over chemical pesticides for S. frugiperda control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
This study investigated the effect of commonly used biopesticides on Spodoptera frugiperda management and its larval parasitoids in Ghana, in comparison with a chemical insecticide based on emamectin benzoate and acetamiprid and untreated control.
-
The diversity and evenness indexes of the parasitoid species did not vary among treatments, but parasitism rates were significantly lower with the chemical on-station.
-
The highest larval density and cob damage and the lowest yields were recorded in the untreated maize plots while, the biopesicides and the chemical treatments displayed similar cob damage and yields.
-
Biopesticides should be preferred over chemical pesticides for S. frugiperda control.
Introduction
The fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) is a highly destructive pest of cereals, native to tropical and subtropical regions of the Americas (Kenis et al. 2022). The pest invaded Africa, parts of Asia and Australia over the last six years, threatening the food security and income situation of millions of farmers, many of which are small holders relying on maize as their main staple crop (Rwomushana et al. 2018). In addition, the pest, known to be highly polyphagous, is likely to jeopardize the trade and export of other crops from the invaded regions.
Following the invasion of S. frugiperda into Africa, emergency responses were geared towards the use of chemical insecticides. The severity of S. frugiperda infestation resulted in farmers repeatedly spraying insecticides during the cropping season, often relying on broad spectrum active ingredients of high toxicity, especially in the first years following the outbreak (Tambo et al. 2020a). The frequent application of broad-spectrum insecticides, however, may be unsustainable because it increases production costs, risk for the development of insecticide resistance, health risks to the growers and consumers, as well as impacts on biodiversity and the environment (Pimentel and Andow 1984; Yu 1991; Togola et al. 2018). Broad spectrum pesticides further have high potential to disrupt natural biological control in maize fields, which, in Africa and other regions, are mostly not or hardly treated with insecticides (Hruska 2019).
There are over 150 parasitoid species that are reported to parasitize S. frugiperda from its native range in the Americas (Molina-Ochoa et al. 2003). Studies conducted in Africa have recently shown over 27 species of parasitoids and the numbers are likely to increase due to species being overlooked and others that will still need to adapt to this new invasive pest (Laouali et al. 2018; Sisay et al. 2019; Agboyi et al. 2020; Laminou et al. 2020; Durocher-Granger et al. 2021; Otim et al. 2021; Abang et al. 2021). Similarly, studies in China and India have shown a considerable number of natural enemies, both looking at predators and parasitoids (Firake and Behere 2020). Already in the first or second year after invasion of fall armyworm, combined parasitism and predation rates were reported to exceed 50% in some locations, suggesting that the local community of natural enemies could play a significant role in controlling this invasive pest (Kenis et al. 2022). However, the use of highly hazardous and broad-spectrum insecticides might threaten these local natural enemies and it is important to minimize their use by developing, promoting and deploying proven and sustainable biopesticides against S. frugiperda, under Integrated Pest Management (IPM) strategies.
Several biopesticides are available for control of S. frugiperda, for instance, the oil extracted from seeds of the neem tree (Azadirachta indica L.) has recently been shown to be effective and even relatively cheap at controlling this pest (Babendreier et al. 2020). Products based on Bacillus thuringiensis (Bt) have also been shown to be effective and generally a considerable number of biopesticides are available in African countries, with numbers increasing over the last few years (Bateman et al. 2021).
In Ghana, the Ministry of Food and Agriculture initiated a programme to promote the use of biopesticides for the control of this pest by smallholder farmers since 2018, subsidizing in particular products based on Bacillus thuringiensis, Azadirachtin and Maltodextrin (vegetable oil and starch) as active ingredients (Asare-Nuamah 2020; Tambo et al. 2020a). These products are reported to be effective against S. frugiperda by farmers, extension agents and researchers, however, there is no information about their compatibility with natural enemies of S. frugiperda occurring in the country and reported in our previous study (Agboyi et al. 2020). The objectives of this study were to assess the effectiveness of biopesticides for S. frugiperda management but in particular their effect on locally present parasitoids of S. frugiperda in northern Ghana, and to identify the most sustainable products compatible with conservation and augmentation biocontrol of S. frugiperda.
Materials and methods
Study sites
This work was conducted in the Guinea Savannah zone of northern Ghana from June to November 2020. The climate in this zone is classified as tropical savannah climate with non-seasonal or dry-winter characteristics (Geiger 1961) and annual daily maximum temperatures of between 26 and 45 °C. The region has a uni-modal rainfall pattern that starts in May and ends in October, followed by a dry season from November to April (mean annual rainfall is about 1100 mm). The soil in the experimental area belongs to the Savannah Ochrosol type, with a relatively thin layer of top soil (about 25 cm deep) consisting of greyish brown loamy sand.
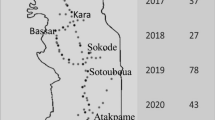
Both on-station and on-farm trials were undertaken. The on-station studies were implemented at the research fields belonging to the Council for Scientific and Industrial Research-Savanna Agricultural Research Institute (CSIR-SARI) near Wa (Longitude: −2.5058; Latitude: 10.0783) in Upper West region and Nyankpala (Longitude: −0.9899; Latitude: 9.4022) in Northern region. The on-farm studies were conducted on farmers’ fields in Wa (Longitude: −2.4281; Latitude: 10.0891) (Fig. 1).
Experimental design
On-station trial
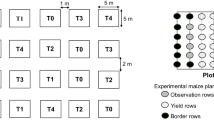
At Wa and Nyankpala stations, the experimental design was a randomized complete block design (RCBD) with 5 replications each. The plots measuring 4.5 m × 5 m were separated by 2 m alleys within a block while blocks were 3 m apart. At both sites, the fields were tractor ploughed followed by harrowing. The maize variety used was Sansalsima, a drought tolerant variety commonly planted in northern Ghana. It was planted at a spacing of 75 cm between rows and 40 cm between plants within a row, with two seeds per hole. The sowing dates were 29 June and 21 July 2020 for Wa and Nyankpala, respectively. Treatments included four biopesticides, one synthetic insecticide (positive control) and an untreated control (see Table 1 for treatments and application rates). Treatment applications were carried out two weeks after crop emergence, and were repeated at three weeks intervals thereafter, with three applications in total. All treatments were applied early in the morning, using CP–15 knapsack sprayers (capacity: 15 l). One sprayer was used for the synthetic insecticides spraying and another one for spraying biopesticides. The latter was thoroughly cleaned between application of the different biopesticides. The whole maize plants were sprayed, during the treatment. In order to reduce product drift, a physical barrier made up of a plastic tarpaulin was placed around each given plot prior to the application of insecticides. The volume of spray solution used during the first, second and third spray applications was 0.5 l, 1 l and 2 l, respectively, for a total of 5 plots (replications) for each treatment.
In this experiment, 250 kg/ha of granular fertilizer, NPK (23-10-10), were placed in holes dibbled near the maize plants as basal fertilizer, two weeks after germination. This was followed by top dressing with 125 kg/ha urea (46% N), 4 weeks after the basal fertilization. All plots were hand weeded twice before the maize ear filling stage. No other crop management or plant protection measure was applied in the plots until harvesting.
On-farm trial
A total of 42 maize farms located in seven communities (6 maize farms per community) in the Upper West region were selected and georeferenced for this on-farm study (Fig. 1). All fields were tractor ploughed and harrowed before sowing. In each community, the maize farms belonged to six farmers who agreed to collaborate. They each offered a field measuring at least one acre and the minimum distance between the maize fields was 50 m. The farmers selected in each community were randomly supplied with one of the five insecticides (biopesticides/synthetic insecticide) to be tested (Table 1). All fields, including the control plots, were established between 29 June and 13 July 2020 using the maize variety Sansalsima and all treatments were applied as described above for the on-station trials by farmers with the assistance of technical advisors (Table 1).
Data collection
Initial background data were collected from all plots two weeks after crop emergence, directly before the first treatment. For the on-station trials, data were collected six days after the first treatments, one day before the second and the third application of treatments and six days after the third application date, respectively, so five times in total. For the on-farm studies, the farms were sampled fortnightly starting again directly before treatments were applied for the first time, and five times in total (Fig. 2).
Data were collected from 30 randomly selected plants per plot following a “W” pattern for the two on-station trials and on 50 plants per farm following a “W” pattern for the on-farm studies where plots were larger. For all plots, data were collected by staff from CSIR—SARI. During data collection, the plants were carefully inspected for the presence of S. frugiperda larvae. All S. frugiperda larvae present were counted but only younger ones (L1-L3) were carefully removed from the plant and transferred into ventilated plastic cups (V: 80 ml) containing some tissue paper to assess the diversity and the relative abundance of larval parasitoids. Only young instars were collected because earlier studies (Agboyi et al. 2020) showed that all the abundant larval parasitoids of S. frugiperda in Ghana are koinobiont hymenopteran parasitoids attacking eggs and young larvae. The S. frugiperda larvae collected were kept in the same cups in the laboratory under room conditions (average temperature: 27 °C; average relative humidity: 76%; photoperiod: 12 h light:12 h dark) and checked daily for emergence of parasitoids. Larvae were provided with maize leaves collected from 3–4 weeks old untreated maize until either the emergence of parasitoids or S. frugiperda moth. Parasitoid adults that emerged from the samples were preserved in 96% ethanol and identified by comparison with barcoded voucher specimens deposited in GenBank (Agboyi et al. 2020; Durocher-Granger et al. 2021). New species not included in the lists were identified based on the morphology by the author MK. The relative abundance of the parasitoid species was calculated by dividing the number of individuals of each parasitoid species by the total number of parasitoids obtained from the sample collected and expressing this value as a percentage. Parasitism rates were calculated by dividing the number of parasitized larvae by the sum of the parasitized larvae and unparasitized larvae reaching the pupal stage (Agboyi et al. 2020).
Crop damage caused by S. frugiperda was assessed on 30 and 50 maize plants on-station and on-farm, respectively, using the Davis and Williams (1992) damage score scale (from 0—no damage, to 9-very heavy damage). Cob damage was assessed for the on-station trials by selecting 10 cobs at random from each plot, while 30 cobs were selected from the on-farm plots. The cobs were separated into damaged and undamaged cobs; damaged cobs were those that had symptoms of S. frugiperda larvae feeding on the grains. Cob damage was computed as a proportion of the number of cobs damaged from the total number of cobs observed.
Yield estimates for the farmer fields were generated by first demarcating a 10 m × 10 m area in the centre of each of the maize plots. All maize cobs within that 100 m2 area were harvested, dried, threshed, winnowed and the grains were weighed. For the on-station trials, all maize cobs from the inner four rows of the plots (area: 15 m2) were harvested and treated like those from the farmer fields. The grain weight from each plot was converted into yield per ha.
Data analysis
Parasitoids species diversity was analysed by calculating the Shannon’s diversity index (H') and Pielou evenness index (E). The Shannon’s diversity index is function of the population proportions of all species. It is the population value of the average diversity and it is calculated by using the following formula:
where qi = the number of individuals in the ith specie, and \(\mathrm{Q}={\sum }_{i=1}^{s}{q}_{i}\)
The Pielou evenness index was used to assess the parasitoid species population evenness in the parasitoid community. It is calculated using the formula:
where H′ = Shannon’s diversity index, S = total number of species in the community.
Data on fall armyworm infestation levels, maize plant damage and larval parasitism rates were analysed using generalized linear models with a binary logistic response variable (i.e. coding parasitized larvae as ‘1’ and unparasitized larvae as ‘0’) and the Logit link function. This was conducted separately for the three different sites and not including data of the first sampling date which was done before the treatments were applied. An overall analysis (again based on binary generalized linear models) was conducted to compare parasitism rates among the three sites. Means were separated using LSD tests at 5% probability threshold.
Data on cob damage and maize grain yield were analysed using ANOVA, with means separated based on Tukey’s HSD post-hoc tests and a 5% probability threshold. All data were analysed using SPSS Statistics 27.
Results
Larval parasitoid relative abundance
The species Coccygidium luteum (Brulle) (Hymenoptera: Braconidae) and Chelonus bifoveolatus Szépligeti (Hymenoptera: Braconidae) were the most abundant species occurring in Wa, both on-farm and on-station, followed by Charops sp. (Hymenoptera: Ichneumonidae), Cotesia icipe Fernandez-Triana and Fiaboe (Hymenoptera: Braconidae) and Meteorus sp. (Hymenoptera: Braconidae) (Table 2). The species Chelonus curvimaculatus Cameron (Hymenoptera: Braconidae) and Drino sp. (Diptera: Tachinidae) were rarely collected in Wa. In the on-station trial in Nyankpala, C. luteum, was the most abundant parasitoid, followed by Meteorus sp., Ch. bifoveolatus, Charops sp., Drino sp. and C. icipe in decreasing order. Chelonus curvimaculatus was absent from the samples collected from different treatments in Nyankpala (Table 2). No statistical analysis was conducted on these abundance data because of low numbers in some of the species and also because of a bias due to the difference in the number of hosts per treatment, reflecting different levels of efficacy of the pesticides tested.
Larval parasitoid species diversity and evenness
In general, the values of Shannon’s diversity index were low and varied between sites but were overall similar between treatments. Regardless of the treatments, lower values of the Shannon’s diversity index were recorded on-station in Wa (0.46 ≤ H′ 0.67), compared to the on-farm trial in Wa (0.89 ≤ H′ ≥ 1.08) and the on-station trial in Nyankpala (0.85 ≤ H′ ≥ 1.48) (Table 3). No consistent effects could be observed; however, the biopesticide Agoo and the negative control plot displayed higher Shannon’s diversity index values in Nyankpala when compared to the chemical positive control Ema Star. The Pielou evenness index values were similar between the treatments at the different sites and ranged between 0.28–0.42, 0.64–0.88 and 0.71–0.92 on-station and on-farm in Wa and on-station in Nyankpala, respectively (Table 3).
Effect of insecticides on larval parasitism of S. frugiperda
A total of 2482 S. frugiperda larvae were collected over five sampling dates for the on-station trial in Wa. Of these, 242 larvae died in the laboratory from unknown cause and 481 were parasitized resulting in an overall pooled parasitism rate of 21.5% (Table 4). Differences were found among treatments in parasitism rate for the on-station trials in Wa (χ25,15 = 12.2, p = 0.033). This was mostly driven by significantly lower parasitism rates in the Ema Star treatment when compared to all other treatments except Grow Safe (all p < 0.05). Significant differences were also observed among the different dates (χ23,15 = 77.6, p < 0.001).
From the 42 farmer plots sampled on-farm in Wa, 3,266 larvae were collected for parasitism assessment, of which 368 died before reaching pupal stage due to unknown reasons. The overall pooled parasitism rate for farmer’s fields was 9.4% (Table 4) and no difference was found among treatments in parasitism rates (χ25,15 = 2.22, p = 0.82). Similar to Wa on station, significant differences were observed among the different dates (χ23,15 = 39.3, p < 0.001).
In Nyankpala, a total of 3,163 larvae were collected of which 485 died due to unknown reasons and 395 of them were parasitized. The overall mean larval parasitism rate in Nyankpala was 14.7%. Significant differences in parasitism rate were found among treatments (χ25,15 = 14.1, p = 0.015; Table 4) which were due to significantly lower parasitism rates in the Bypel 1 treatment when compared to all other treatments (all p < 0.005). Significant differences were again observed among the different dates (χ23,15 = 39.2, p < 0.001).
Significant effects were found among the three locations (F2,61 = 206.5, p < 0.001) with parasitism rates being significantly higher in Wa on-station compared to Nyankpala on-station (p < 0.001) and higher in Nyankpala on-station compared to Wa on-farm trials (p < 0.001).
Effect of insecticides on larval numbers, plant and cob damage and yield
Highly significant differences were found among the different pesticides in terms of the number of S. frugiperda larvae collected (F5,402.501 = 11.9, p < 0.001). All treatments significantly reduced the number of larvae compared to the control (p < 0.018). The number of larvae found in the Bypel and Ema Star treatments was significantly lower than in the other three biopesticides treatments (p < 0.012, see Table 5). Slightly significant differences were also found among the three locations (F2,402.810 = 3.59, p = 0.029) with Wa on-station showing slightly lower numbers of S. frugiperda larvae compared to Nyankpala on-station (p = 0.029) and Wa on-farm (p = 0.013), whereas no difference in larval numbers were found between Nyankpala on-station and Wa on farm (p = 0.90).
Significant differences among treatments were also found for maize plant damage caused by S. frugiperda (F5,171,499 = 3.45, p = 0.005). In line with the results obtained for larvae, the lowest damage rates were found in the Bypel and Ema Star treatments (Table 6).
Regarding cob damage, highly significant differences were found among treatments in a global analysis over all sites (F5,74 = 13.3, p < 0.001) (Fig. 3a). Cob damage rates were significantly higher in the control than in all other treatments (p < 0.05 for all comparisons). In addition, the positive control Ema Star showed significantly lower cob damage levels than Agoo (p = 0.044), Eradicoat (p = 0.002) and Grow Safe (p = 0.048). Significant differences in the proportion of damaged cobs were also found among locations (F2,74 = 10.8, p < 0.001) with lower cob damage rates in Nyankpala compared to Wa on-station and Wa on-farm (p < 0.001). For cob damage, a significant interaction was obtained between the pesticide treatments and locations (F10,74 = 3.57, p < 0.001).
Mean maize cob damage rates (± SD) (top) and mean maize grain yield (bottom) caused by S. frugiperda under different pesticide treatments on-station (n = 5) and on-farm in Northern Ghana (n = 7). Treatments showing the same letter above bars are not significantly different from each other for a given site (Tukey’s HSD post-hoc tests; p < 0.05)
Finally, highly significant differences were found among treatments for maize grain yield (F5,72 = 8.4, p < 0.001). Results were mainly driven by significantly lower yields in the control as compared to all other treatments, especially on-station (Fig. 3b). Yield was significantly different among locations (F2,72 = 264.8, p < 0.001) with higher yields found for Wa on-station when compared to Nyankpala and Wa on-farm (p < 0.001).
Discussion
Chemical pesticide applications against S. frugiperda have been recommended in most invaded areas, resulting in various levels of control. However, it is a well-known fact that the use of chemical pesticides may have serious health and environmental impacts (PAN UK 2007; Miah et al. 2014), including on beneficial organisms such as natural enemies relevant for pest control (Pimentel 1995; Desneux et al. 2007). On the other hand, there is general belief that biopesticides are less harmful to natural enemies, although evidence from field studies on S. frugiperda is lacking (Kenis et al. 2022). In this study, we assessed the impact of commonly used biopesticides recommended and distributed by the government of Ghana to farmers against S. frugiperda on the local parasitoids associated with this pest, in comparison with the most used chemical pesticide, Ema Star, and a negative control. In general, parasitism was lower in the fields treated with Ema Star and Bypel 1 as compared to untreated plots and those treated with the other biopesticides, although the tendency was not consistently significant throughout the sites and dates. This may be due to the fact that the parasitoid complex varied with sites and dates and it well known that different parasitoid species may react differently to pesticide and biopesticide treatments. For example, it was shown in Florida that the braconid egg-larval parasitoid Chelonus insularis Cresson was much less present in maize fields sprayed with insecticides than in untreated fields, which was not the case for the larval parasitoid Cotesia marginiventris (Cresson) (Meagher et al. 2016). Another possible reason for the relatively low impact of pesticides and biopesticides on parasitism is the size of the plots and the ability of parasitoids to quickly move from adjacent, untreated fields or vegetation to the previously treated plots. The three-week interval between applications may be long enough to allow for significant restoration of the parasitoid populations after each treatment. The parasitoid species attacking S. frugiperda in Ghana are known to parasitize other Lepidoptera in many agroecosystems (Agboyi et al. 2020; Koffi et al. 2020). At plot level, the use of pesticides against a pest is probably more detrimental to less mobile natural enemies such as non-flying predators (earwigs, ants, spiders, etc.), whereas more mobile parasitoids are likely more affected by massive uses of pesticides at landscape scale during a longer period.
Furthermore, the insecticide used in this study, Ema Star, is surely not the most detrimental to natural enemies. Its active ingredients, emamectin benzoate and acetamiprid, are both classified as moderately hazardous (Class II) technical grade active ingredients (WHO 2019), whereas, in Africa, a whole list of more hazardous pesticides are used against S. frugiperda (Tambo et al. 2020b). The WHO class I products are phased out in many important markets such as the EU and pressure is high also on the class II products, e.g. for most neonicotinoids. WHO class II products are also generally aimed to be reduced or avoided as much as possible in different types of IPM standards. Still, the effects of emamectin benzoate and acetamiprid on natural enemies may be less severe than expected based solely on the WHO class system. For instance, Sechser et al (2003) found a relatively low hazard of emamectin benzoate on natural enemies, after studying its effects on predators of sucking pests of cotton. For acetamiprid, after assessing the risk quotient for different chemicals belonging to the neonicotinoid family, Jiang et al. (2019) considered that acetamiprid was the only relatively safe neonicotinoid insecticide for the egg parasitoids Trichogramma spp. Acetamiprid was also found to have low effect on some predators’ population dynamics, such as the true bug Orius sauteri (Poppius) (Lin et al. 2020).
It is unclear how a product such as Bypel 1, based on Pieris rapae granulosis virus and Bt, could potentially affect parasitoids, other than through an indirect effect via the mortality of their host. Although the effect was not consistent among sites, there is probably a need for further studies to clarify the effect that such a biopesticide formulation has on parasitoids.
Maize sprayed thrice with Bypel 1 or Ema Star also induced the strongest reductions in S. frugiperda larval density, which probably leads to a reduction in cues that attract female parasitoids present in the area. This potentially leads to a density dependence of the parasitism rate in S. frugiperda larvae, as observed in Zambia (Durocher-Granger et al. 2021). The density dependence of parasitism on S. frugiperda needs to be further assessed.
The untreated plots showed the highest larval density and plant damage, the highest cob damage, and generated the lowest yields, suggesting that the treatments were at least partly efficient to control the pest. There was no consistent difference in cob damage and yields among the chemical pesticide and the biopesticides. In such situations, biopesticides should be preferred to minimize negative effects on human health, natural control and the environment in general.
Author contributions
LKA, DB and MK conceptualized the work. LKA, DB and MK wrote the methodology. JAN, EA, BKB, LKA and PB conducted the trials. DB analysed the data. LKA, DB and JAN prepared the original draft. All authors read, reviewed and approved the manuscript.
Data availability and materials
The datasets generated in this study are available from the corresponding author on reasonable request.
References
Abang AF, Nanga SN, FotsoKuate A, Kouebou C, Suh C, Masso C, Saethre M-G, Fiaboe KKM (2021) Natural enemies of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in different agro-ecologies. Insects 12:509. https://doi.org/10.3390/insects12060509
Agboyi LK, Goergen G, Beseh P, Mensah SA, Clottey VA, Glikpo R, Buddie A, Cafà G, Offord L, Day R, Rwomushana I, Kenis M (2020) Parasitoid complex of fall armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 11:68. https://doi.org/10.3390/insects11020068
Asare-Nuamah P (2020) Smallholder farmers’ adaptation strategies for the management of fall armyworm (Spodoptera frugiperda) in rural Ghana. Int J Pest Manage 68(1):8–18. https://doi.org/10.1080/09670874.2020.1787552
Babendreier D, Agboyi LK, Beseh P, Osae M, Nboyine J, Ofori SEK, Frimpong JO, Clottey VA, Kenis M (2020) The efficacy of alternative, environmentally friendly plant protection measures for control of fall armyworm, Spodoptera Frugiperda, in Maize. Insects 11:240. https://doi.org/10.3390/insects11040240
Bateman ML, Day RK, Rwomushana I, Subramanian S, Wilson K, Babendreier D, Luke B, Edgington S (2021) Updated assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. J Appl Entomol 145:384–393. https://doi.org/10.1111/jen.12856
Davis FM and Williams WP (1992) Visual rating scales for screening whorl stage corn for resistance to fall armyworm. Mississippi Agricultural and Forestry Experiment Station, Technical Bulletin 186, Mississippi State University, MS39762, USA
Desneux N, Decourtye A, Delpuech J (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Durocher-Granger L, Mfune T, Musesha M, Lowry A, Reynolds K, Buddie A, Cafà G, Offord L, Chipabika G, Dicke M, Kenis M (2021) Factors influencing the occurrence of fall armyworm parasitoids in Zambia. J Pest Sci 94:1133–1146. https://doi.org/10.1007/s10340-020-01320-9
Firake DM, Behere GT (2020) Natural mortality of invasive fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae) in maize agroecosystems of northeast India. Biol Control 148:104303. https://doi.org/10.1016/j.biocontrol.2020.104303
GEIGER R (1961) Uberarbeitete Neuausgabe von Geiger R: K¨oppen-Geiger / Klima der Erde. (Wandkarte 1:16 Mill.) – Klett-Perthes, Gotha
Hruska AJ (2019) Fall armyworm (Spodoptera frugiperda) management by smallholders. CAB Rev 14:043. https://doi.org/10.1079/PAVSNNR201914043
Kenis M, Benelli G, Biondi A, Calatayud PA, Day R, Desneux N, Harrison RD, Kriticos D, Rwomushana I, van den Berg J, Verheggen F, Zhang YJ, Agboyi LK, Ahissou RB, Ba MN, Bernal J, de Freitas Bueno A, Carrière Y, Carvalho GA, Chen XX, Cicero L, du Plessis H, Early R, Fallet P, Fiaboe KKM, Firake DM, Goergen G, Groot AT, Guedes RNC, Gupta A, Hu G, Huang FN, Jaber LR, Malo EA, McCarthy CB, Meagher RL Jr, Mohamed S, Mota Sanchez D, Nagoshi RN, Nègre N, Niassy S, Ota N, Nyamukondiwa C, Omoto C, Reddy Palli S, Pavela R, Ramirez-Romero R, Rojas JC, Subramanian S, Tabashnik BE, Tay WT, Virla EG, Wang S, Williams T, Zang LS, Zhang L, Wu K (2022) Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol Gen. https://doi.org/10.1127/entomologia/2022/1659
Koffi D, Kyerematen R, Eziah VY, Agboka K, Adom M, Goergen G, Meagher RL (2020) Natural enemies of the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in Ghana. Fla Entomol 103:85–90. https://doi.org/10.1653/024.103.0414
Laminou SA, Ba MN, Karimoune L, Doumma A, Muniappan R (2020) Parasitism of locally recruited egg parasitoids of the fall armyworm in Africa. Insects 11(7):430. https://doi.org/10.3390/insects11070430
Laouali A, Ibrahim B, Ba MN, Laouali K, Rangaswamy M (2018) Native parasitoids recruited by the invaded fall army worm in Niger. Indian J Entomol 80(4):1253–1254. https://doi.org/10.5958/0974-8172.2018.00338.3
Lin R, He D, Men X, Zheng L, Cheng S, Tao L, Yu C (2020) Sublethal and transgenerational effects of acetamiprid and imidacloprid on the predatory bug Orius sauteri (Poppius) (Hemiptera: Anthocoridae). Chemosphere 255:126778. https://doi.org/10.1016/j.chemosphere.2020.126778
Meagher RL, Nuessly GS, Nagoshi RN, Hay-Roe MN (2016) Parasitoids attacking fall armyworm (Lepidoptera: Noctuidae) in sweet corn habitats. Biol Control 95:66–72. https://doi.org/10.1016/j.biocontrol.2016.01.006
Miah SJ, Hoque A, Paul A, Rahman A (2014) Unsafe use of pesticide and its impact on health of farmers: a case study in Burichong Upazila, Bangladesh. J Environ Sci Toxicol Food Technol 8(1):57–67. https://doi.org/10.9790/2402-08155767
Molina-Ochoa J, Carpenter JE, Heinrichs EA, Foster JE (2003) Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: an inventory. Fla Entomol 86:254–289. https://doi.org/10.1653/0015-4040(2003)086[0254:PAPOSF]2.0.CO;2
Otim MH, AdumoAropet S, Opio M, Kanyesigye D, NakeletOpolot H, TekTay W (2020) Parasitoid distribution and parasitism of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in different maize producing regions of Uganda. Insects 12:121. https://doi.org/10.3390/insects12020121
Pimentel D (1995) Amounts of pesticides reaching target pests: environmental impacts and ethics. J Agric Environ Ethics 8:17–29. https://doi.org/10.1007/BF02286399
Pimentel D, Andow DA (1984) Pest management and pesticide impacts. Int J Trop Insect Sci 5(03):141–149. https://doi.org/10.1017/s1742758400008201
Rwomushana I, Bateman M, Beale T, Beseh P, Cameron K, Chiluba M, Clottey V, Davis T, Day R, Early R, Godwin J, Gonzalez-Moreno P, Kansiime M, Kenis M, Makale F, Mugambi I, Murphy S, Nunda W, Phiri N, Pratt C, Tambo J Fall Armyworm: Impacts and Implications for Africa. CABI Evidence Note Update, CABI, Wallingford, UK
Sechser B, Ayoub S, Monuir N (2003) Selectivity of emamectin benzoate to predators of sucking pests on cotton. J Plant Dis Prot 110(2):184–194. https://www.jstor.org/stable/43215501
Sisay B, Simiyu J, Mendesil E, Likhayo P, Ayalew G, Mohamed S, Subramanian S, Tefera T (2019) Fall armyworm, Spodoptera frugiperda infestations in East Africa: Assessment of damage and parasitism. Insects 10:195. https://doi.org/10.3390/insects10070195
Tambo JA, Day RK, Lamontagne-Godwin J, Silvestri S, Beseh PK, Oppong-Mensah B, Noah AP, Matimelo M (2020a) Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: an analysis of farmers’ control actions. Int J Pest Manag 66(4):298–310. https://doi.org/10.1080/09670874.2019.1646942
Tambo JA, Kansiime MK, Mugambi I, Rwomushana I, Kenis M, Day RK, Lamontagne-Godwin J (2020b) Understanding smallholders’ responses to fall armyworm (Spodoptera frugiperda) invasion: evidence from five African countries. Sci Total Environ 740:140015. https://doi.org/10.1016/j.scitotenv.2020.140015
Togola A, Meseka S, Menkir A, Badu-Apraku B, Bouka O, Tamò M, Djouaka R (2018) Measurement of pesticide residues from chemical control of the invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a maize experimental field in Mokwa, Nigeria. Int J Environ Res Public Health 15:849. https://doi.org/10.3390/ijerph15050849
PAN UK (2007) Hazardous pesticides and health impacts in Africa. Food & Fairness Briefing, 6.
WHO (2019) WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition. Geneva, World Health Organization, 2020, Licence: CC BY-NC-SA 3.0 IGO.
Yu SJ (1991) Insecticide resistance in the fall armyworm, Spodoptera frugiperda (JE Smith). Pestic Biochem Physiol 39(1):84–91. https://doi.org/10.1016/0048-3575(91)90216-9
Acknowledgements
We thank all the technicians from the Savanna Agricultural Research Institute (SARI) for their assistance in collecting the data and the farmers for their collaboration.
Funding
The research was financially supported by the Foreign, Commonwealth and Development Office (FCDO), UK, the Directorate-General for International Cooperation (DGIS), the Netherlands, the European Commission Directorate-General for International Cooperation and Development (DEVCO) and the Swiss Agency for Development and Cooperation (SDC) through CABI’s Action on Invasives and Plantwise Plus Programmes. CABI is an international intergovernmental organisation and we gratefully acknowledge the core financial support from our member countries and lead agencies. See https://www.cabi.org/aboutcabi/who-we-work-with/key-donors/ for details.
Author information
Authors and Affiliations
Contributions
LKA, DB and MK conceptualized the work. LKA, DB and MK wrote the methodology. JAN, EA, BKB, LKA and PB conducted the trials. DB analysed the data. LKA, DB and JAN prepared the original draft. All authors read, reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Competing interests
The authors declare no competing interests.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Communicated by Gaelle Le Goff.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agboyi, L.K., Nboyine, J.A., Asamani, E. et al. Comparative effects of biopesticides on fall armyworm management and larval parasitism rates in northern Ghana. J Pest Sci 96, 1417–1428 (2023). https://doi.org/10.1007/s10340-023-01590-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01590-z