Abstract
The Walnut Twig Beetle (WTB), Pityophthorus juglandis Blackman, is a small bark beetle native to Mexico and Southwestern USA recorded for the first time in Europe (NE Italy) in 2013. WTB attacks walnut (Juglans spp.) and wingnut trees (Pterocarya spp.) and is the vector of Geosmithia morbida Kolarík et al., a pathogen causing the thousand cankers disease (TCD). WTB and TCD represent a serious threat for walnut orchards in Europe. Spatiotemporal data of the WTB-TCD infestations recorded from an 8-year-long (2013–2020) monitoring conducted in 106 walnut orchards of NE Italy were used to develop a model in order to analyze: (i) the effective dispersal capacity of WTB, (ii) the factors affecting dispersal and (iii) the colonization risk of healthy walnut orchards. We registered a mean annual dispersal of 9.4 km, with peaks of about 40 km. Pest dispersal is affected by distance of suitable hosts from the nearest infested site, number of walnut orchards in the surroundings (both infested and healthy), orchard size and walnut species in the orchard. Using the model, it was also possible to calculate the colonization risk of a specific walnut orchard according to its characteristics showing, for instance, that a medium-size (5,000 trees) black walnut orchard located at 25 km from the nearest infested orchard has an infestation risk of about 50% of probability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Pityophthorus juglandis is a Neartic pest of walnut trees introduced in Europe in 2013.
-
No study verified the WTB spread capacity for Europe and the factors affecting the dispersal.
-
This lack makes effective containment and eradication measures difficult to plan.
-
We found that even at distances greater than 50 km the risk of colonization can be high.
-
The risk depends on the number of orchards in the surroundings, their size and walnut species.
Introduction
International trade, moving large amounts of goods globally, leads to inadvertent global translocation of invasive insects (Seebens et al. 2018). Wood-boring beetles, like bark and ambrosia beetles (Scolytinae), longhorn beetles (Cerambycidae) and jewel beetles (Buprestidae), are one of the most successful guilds of alien species invasive of forest habitats (Eyre and Haack 2017). Bark and ambrosia beetles, especially, represent a serious threat to European forests and wood orchards, due both to the ease with which they are transported all over the world inside wood-packaging materials, timber and woody plants (Meurisse et al. 2019), and the plant pathogens they may carry (Kirisits 2007; Six 2012; Ploetz et al. 2013; Carrillo et al. 2014; Malacrinò et al. 2017). Every year, new alien species of bark and ambrosia beetles are recorded in Europe (Kirkendall and Faccoli 2010; Barnouin et al. 2020). An example is the Walnut Twig Beetle (WTB), Pityophthorus juglandis Blackman (Coleoptera: Curculionidae, Scolytinae), found for the first time in Europe in 2013 in northeastern Italy (Montecchio and Faccoli 2014).
Pityophthorus juglandis is a small (1.5–1.9-mm-long) bark beetle native to Mexico and southwest USA (Wood and Bright 1992). Adults colonize and reproduce in the phloem of walnut (Juglans spp.) and wingnut trees (Pterocarya spp.). In spring, with a mean air temperature of about 18 °C, overwintering adults disperse and infest new hosts mainly at the base of twigs, although large branches and the warmer side of the trunk can also be attacked (Newton and Fowler 2009). In southern Europe, the first-generation development takes about 7–9 weeks with new adult emergence occurring at mid-end July, whereas the second generation is complete at the end of September (Faccoli et al. 2016). In both North America and Europe, P. juglandis is the vector of Geosmithia morbida Kolarík et al., an aggressive pathogen causing the thousand cankers disease (TCD) (Kolařík et al. 2011). The conidia of the pathogen are carried on the elytra of WTB adults (Newton and Fowler 2009) and introduced into the host plant during beetle bark colonization (Tisserat et al. 2009).
Pityophthorus juglandis is native to Mexico, New Mexico, Arizona and Southern California (Bright and Stark 1973; Wood and Bright 1992). Nevertheless, since 2009 WTB and the associated pathogen G. morbida have spread to northwestern USA (Colorado, Idaho, Oregon, Utah and Washington) and the east coast, reaching Tennessee (2010), Nevada (2011), Virginia (2011), Pennsylvania (2011), Ohio (2012), North Carolina (2012), Indiana (2013) and Maryland (2013) (Cranshaw 2011; Seybold et al. 2012, 2013; Wiggins et al. 2014; Rugman-Jones et al. 2015).
In 2013, the species crossed the Atlantic Ocean reaching Europe, where both P. juglandis and G. morbida were found in a black walnut orchard in Vicenza province (Veneto region, northeastern Italy) (Montecchio and Faccoli 2014; Montecchio et al. 2014). During a preliminary visual survey conducted immediately in the surrounding walnut orchards, a further four infested sites (i.e., orchards) were found in the same province (Faccoli et al. 2016). The following year (2014), a second more extensive survey was done in the whole Veneto region (where the first finding was recorded) and in the two west and east bordering regions (Lombardy and Friuli-Venezia Giulia), to check the real distribution of WTB in northeastern Italy. During the second survey, the pest was found in other nine orchards, including one located in Lombardy (Faccoli et al. 2016). Since 2013, WTB has spread in many other regions of central-northern Italy, and to date, the species has been recorded in Friuli-Venezia Giulia (2015) (Montecchio et al. 2016), Piedmont (2015) (Bosio and Cooke-McEwen 2018), Tuscany (2018) (Moricca et al. 2019) and Emilia Romagna (2019) (EPPO 2019). The dispersal of P. juglandis and its associated pathogen G. morbida in Italy and Europe is considered a serious threat for walnut orchards (mainly J. nigra) largely used in the last decades for wood production (Eichhorn et al. 2006). Massive attacks of this pest may have great impacts on the economy and landscape of many areas of southern Europe, as occurred in the USA (Leslie et al. 2009; Seybold et al. 2019).
In less than 6 years, WTB has colonized 13 American States and reached another continent, representing one of the fastest and most successful invaders among forest insect species. Nevertheless, little is known about the active and passive dispersal capacity of P. juglandis. In a recent laboratory experiment, Kees et al. (2017) found that the maximum total active flight distance covered by WTB adults was about 3.6 km in 24 h, but mean and median distances flown by beetles were much lower (about 372 m and 158 m, respectively). However, the contribution of natural dispersal (for instance, by wind) to the insect’s spread across the Western USA remains unknown (Cranshaw 2011). A recent study carried out on more than 60 American populations of WTB demonstrated that the expansion of the insect is, in part, facilitated by anthropogenic movement of infested wood (Rugman-Jones et al. 2015), which probably also allowed the pest to reach Europe.
In this study, we present the results of the WTB survey conducted for eight consecutive years in northeastern Italy (2013–2020) since the first discovery of the pest in Europe. The aim of the study is to investigate the annual increase in invaded range of P. juglandis based on data from the yearly survey and to present a descriptive model assessing the spreading capacity of WTB populations according to specific environmental parameters. In particular, we hypothesize that the dispersal of WTB is affected by the presence and distribution of walnut orchards, by the walnut species and by the orchard size, as bigger suitable sites are easier to find and may promote greater proliferation of the pest. Based on the historical epidemiological data collected in NE Italy during the last 8-year survey, this model is intended to quantify (a) the real dispersal capacity of the insect, (b) the factors affecting dispersal and (c) the risk of infestation (probability) of healthy walnut orchards in a given area.
Materials and methods
Monitored areas and survey protocol
The survey of WTB occurrence was conducted in the Veneto region (northeastern Italy) since 2013 when the pest and associated pathogen were found for the first time at Bressanvido (province of Vicenza). In the year of discovery, a first inspection was made on 19 walnut orchards within about 10 km of the infested area. Since 2014, a more extensive survey has been conducted in all the provinces of the Veneto region where walnut orchards occur. Initially, the customer lists of the major forest tree nurseries of the region were used to identify all the main walnut orchards planted in Veneto in the previous 15 years. Then, more than 100 walnut orchards identified from the lists and other public parks and private gardens with walnut trees were monitored annually by visual inspections looking for symptoms of insect colonization or disease infection according to the regional protocol for detection of alien species (Montecchio et al. 2016). In addition, 40–50 orchards (depending on the year) homogeneously distributed in the region were monitored annually also with a 12-black-multifunnel trap (Econex, Murcia, Spain) baited with a 400 mg dispenser of a pheromone specific for WTB (3-methyl-2-buten-1-ol; Contech Enterprises, Delta, BC, Canada; Seybold et al. 2013) (Table 1). The release rate was about 1 mg/day with a temperature of 20 °C and generally doubled with every 5 °C rise in temperature. Average daily temperature in the study area in spring–summer is about 25 °C, so the estimated duration of the blend was about 200 days. One trap per site was set up in the last weeks of July, in the middle of the walnut orchard at about 2 m above the ground, fixed to a tree branch and emptied every second week. Traps were removed in the last week of October, 80–90 days later, assuring that they were active during the main emergence period and dispersal of adults of the second generation (Faccoli et al. 2016). Orchards where the presence of WTB was confirmed by captures with the traps or visual inspection were then excluded from the survey in the following years and replaced with new uninfected ones.
Dataset
The dataset used for the analyses was obtained from 106 sites (walnut orchards, public parks and private gardens) visually inspected or monitored with traps in Veneto since 2013 (Table S1). Data collected through different detection methods were handled in the same way, because they only served to ascertain the presence of WTB in the site. Sites from other Italian regions where the WTB presence was recorded in the following years were not included in the dataset because they were isolated points not belonging to a widespread and intensive monitoring network, and were recorded only following an accidental finding. For each monitored site, identified by a unique code, the beginning of monitoring, spatial coordinates (UTM zone 32 N), number of trees, species composition (J. nigra, J. regia or “mixed”) and year of first attack (from that year onwards the site was considered infected) were reported (Table S1). The dataset was then updated year by year with information on new sites considered infested by WTB according to trap captures and visually detected symptoms. WTB has been added in the EPPO A2 Quarantine Pest list just in 2019 (EPPO 2021), and to date, no clear-cut or pesticide treatments were performed in infested sites. For this reason, an “infected site” of our database was considered infected for all subsequent years.
Statistical analysis
A probabilistic model was constructed through the analysis of the binomial (infested or not infested) individual site’s data using a generalized linear model (GLM) with a probit link function (McCullagh and Nelder 1989). The independent variables included in the model tested were: attack index (ati), available host index (ahi), distance to nearest attacked site (dna), orchard size (number of trees in the site) and walnut species (J. nigra, J. regia or mixed orchards). The dependent variable was the probability (P) that an orchard or group of trees can be attacked by WTB as a function of its characteristics. The dependent variable and the first four independent variables (ati, ahi, dna, orchard size) were considered continuous, whereas walnut species was discrete variable.
The attack index (ati) and the available host index (ahi) were introduced by Favaro et al. (2015) in a similar study on the dispersal of invasive populations of the Asian longhorned beetle, Anoplophora glabripennis Motschulsky (Coleoptera: Cerambycidae). The attack index for site i considers how many sites attacked in the previous years by WTB occur nearby, and is calculated as:
where dij is the distance between site i and each site (j) attacked in the previous years, whereas ca is a constant (see below).
The available host index for site i, instead, considers how many available sites (site with available uninfested hosts) occur nearby, and is calculated as:
where dij is the distance between site i and each monitored non-attacked site (j) in the current year, and ch is a constant. The two indices ati and ahi are calculated for each site and year, for a range of values of c. The constants ca and ch are instead estimated using an iterative approach as they represent a threshold distance: all distances dij greater than c have little influence on the final value of the index; the terms related to shorter distances will strongly affect the final value. In order to obtain a biologically plausible model, the constants ca and ch were set on the basis of the displacement capacity of the insect. Because there is currently no precise information about P. juglandis dispersal, we set a surrogate limit value of 80 km, corresponding to the maximum distance covered actively by the six-toothed bark beetle Pityogenes chalcographus (L.) (Coloeptera: Curculionidae), a similar size bark beetle (Nilssen 1984). This distance was considered a feasible estimate also for the WTB by the expert working group who drew up the EPPO report for P. juglandis (EPPO 2015). The best values of ca and ch were chosen using Akaike’s information criterion (AIC) (Akaike 1974), choosing the couple that minimizes the AIC index. Initially, all possible pairs of ca and ch were tested in the model in a range from 1 to 80,000 m (the threshold value presented), with intervals of 2,000 m. Subsequently, the model was gradually refined by narrowing the range of the two constants around the best value and reducing the dispersal interval.
All the independent variables and their interactions were considered in the preliminary model. During each iteration the least significant variable or interaction, evaluated on the basis of the significance level, was removed from the model. The process continued until all nonsignificant variables were eliminated and all variables left were significant at 1% (P < 0.01). Data were analyzed using the GLM routine in R software (R Core Team 2019).
Results
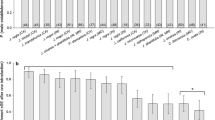
Of the 106 sites monitored over the 8-year survey (2013–2020), 44 walnut orchards (41.5%) were found infested by WTB (Fig. 1). Among the attacked sites, 34 (77%) were black walnut orchards (corresponding to 45% of all black walnut monitored orchards), 6 (14%) were English walnut orchards (corresponding to 29% of all English walnut monitored orchards), and 4 (9%) had a mixed composition. The median size of infested orchards was 92 trees and 25% of attacked sites had at least 160 trees. The year-by-year evolution of the survey, with sites monitored and sites found infected, is shown in Fig. 2.
The cumulative distribution of dna covered annually by WTB indicates a nonlinear trend: the minimum dna registered is about 830 m, whereas the maximum is 40.91 km, with a mean distance of 9.43 ± 0.49 km. However, 25% of newly infested sites were located at least 12.89 km from the closest infested ones (Fig. 3).
All variables considered were significant in the model, even if all the interactions considered were rejected because they did not produce significant effects. The final GLM model, therefore, is the following:
The estimated constants ca and ch for the attack (ati) and available host indexes (ahi) are 21 km and 14 km, respectively. The final GLM fit summary statistic for the model (Eq. 3) is reported in Table 2.
Using the model of Eq. (3), it is possible to predict the attack risk of a healthy walnut orchard as a function of its characteristics and distance from the closest attacked site (Fig. 4). According to the model, the probability of attack of a walnut orchard increases with its size (increasing number of trees) (z-value = 2.803; P < 0.001), but decreases inversely to the distance from the closest infested site (source orchard) (z-value = −4.550; P < 0.001). Moreover, the risk of being infested is higher in orchards composed of J. nigra (Fig. 4a) rather than J. regia (z-value = −4.868; P < 0.001; Fig. 4b), whereas it has an intermediate value for mixed orchards, but still significantly lower than black walnut (z-value = -3.020; P < 0.001).
Discussion
The study analyzes spatial and temporal data related to the dispersal of WTB in northeastern Italy since the species discovery in 2013. Our analysis shows that dispersal of WTB in this region reached up to 41 km in one year, although the probability of new infestations at a given distance depends on the number of walnut orchards occurring around the healthy ones (i.e., attack index and available host index, respectively), its size (i.e., the number of trees), its dominant walnut species (J. nigra or J. regia) and its distance from the sites attacked during the previous year.
The model shows WTB preference for black walnut (J. nigra) over English walnut (J. regia), consistent with the literature (Newton and Fowler 2009; Wilstermann et al. 2020). In the sites monitored during this study, more than 45% of black walnut orchards were attacked by WTB, compared to 29% of English walnut orchards. Even in mixed composition sites, the risk of attack was significantly lower than in pure black walnut orchards, indicating a greater preference of the pest for this species. Black and English walnuts are largely cultivated in Europe both for nuts and noble hardwood timber production (EPPO 2015), and they can be found in most of Europe, apart from northern regions (de Rigo et al. 2016). Moreover, walnut trees are also widely spread as ornamental plants in parks, gardens and street tree lines (Eichhorn et al. 2006). This capillary presence of host plants could therefore facilitate the spread and stabilization of WTB over much of the continent.
Our study clearly shows that the risk of WTB attack increases with orchard size. An orchard of 10,000 black walnuts should be easy to find by dispersing beetles, showing a risk of attack reaching almost 100% when infested sites occur in the immediate proximity and remaining high (about 50%) even at distances greater than 40 km; instead for a small group of trees, the risk of attack is just over 50% even for very short distances (Fig. 4a). For English walnut, the risk of attack is lower but not negligible (Fig. 4b). The size of the source orchard may also have a crucial role moderating insect dispersal. Faccoli et al. (2016) report that an infestation in a walnut orchard can persist for many years, with a progressive increase in the population density of P. juglandis that will then disperse to other nearby walnut orchards looking for new host trees. Thus, a large walnut orchard can sustain a longer infestation with a large WTB population and a higher dispersal probability.
Another interesting result regards the decrease in the infestation risk with increasing availability of potential host trees, i.e., high available host index (ahi). This means that a site in an area with a large number of other host orchards or groups of trees has a lower probability of being infested than an isolated one. This result can be explained by the colonization strategies of bark beetles. Typically, bark beetles feed and breed in recently dead or severely weakened host trees which are located through specific plant volatiles released by dying trees; nevertheless, when no suitable hosts are available, the insects may also attack healthy hosts massively (using aggregation pheromones), to overcome the plant's defenses (Wood 1982; Kausrud et al. 2011). Walnut orchards are usually maintained in healthy and vigorous conditions to maximize wood and nut production. In this context, a large pest dispersal would have little chance of success (with a few individuals attacking vigorous hosts); for this reason, even with many sites available in the area, the insect colonization remains concentrated only in one or a few orchards, in order to maximize the attack effectiveness. Moreover, with a small WTB population density, a greater number of available host sites reduce the risk of each one being colonized.
Finally, data collected during the survey highlight a high spread of WTB, with dispersal capacity of over 40 km per year (the longest distance between an attacked site and the closest infested one recorded in the previous years) and with 75% of newly infested sites located at least 4 km away from the nearest source sites. Considering the results of Kees et al. (2017), which presented an active flight distance covered by WTB estimated at less than 2 km, additional factors other than active dispersal must be considered to explain such a spread of the pest. As previously observed by Seybold et al. (2012), a fundamental contribution to spread of beetle population is made by the human-mediated movement of walnut logs or wood products. Cases have been observed where even the wind has favored the dispersal of WTB (Cranshaw and Tisserat 2012), although this is not considered as the main dispersal mechanism of diffusion of this pest (EPPO 2015). Such a large active and passive dispersal capacity causes many problems for pest management. The containment measures currently applied in the Veneto region were based on the creation of a buffer zone with 2 km range beyond the infested area (Regione del Veneto 2014a, b, 2015). According to our model, a 2 km buffer around the infested site corresponds to an infestation risk higher than 80% and 40% for medium-size orchards (5,000 trees) of black and English walnut, respectively (Fig. 4). This remains, hence, a very high risk of colonization for walnuts growing also outside the buffer area, despite the considerable size of the buffer proposed. Adjustment of buffer zones, with an increase in radius, can be used in synergy with other techniques to achieve greater protection of still healthy orchards. An example are the recent works by Audley et al. (2020a, b) on the repellent effect of some semiochemicals against WTB: volatiles such as (R)-( +)-limonene, trans-conophthorin, (R)-( +)-verbenone and α-pinene can be used to repel the pest from the sites to be protected.
In conclusion, this model has been built using the data obtained from an eight-year-long surveillance program of the main walnut orchards of Veneto region to explain the natural dispersal of WTB in northeastern Italy. Although some of the most important factors affecting WTB presence and its dispersal probability have been clarified (distance from nearest attacked site, attack index, available host index, orchard size and tree species), the role of other variables—such as environmental conditions (e.g., dominant winds) and human-related activities (e.g., main trade routes, volume and type of potentially infected goods)—should be carefully considered to better understand the potential spread of this relatively new invasive pest across Europe and to establish effective containment and local eradication measures. Help in this regard may come from the recent work of Chen et al. (2020) presenting the effects of climatic variables (i.e., precipitation, solar radiation, vapor pressure, air temperature, relative humidity and wind speed) on the flight activity of WTB. Considering also the other Italian regions where WTB was found, after its first record in 2013 the insect traveled over 320 km westwards reaching the Piedmont region in 2014 (Bosio and Cooke-McEwen 2018) and about 200 km southwards reaching the Tuscany region in 2018 (Moricca et al. 2019). These distances are incompatible with our results (320 km in one year to reach Piedmont) or extremely unlikely (a mean of 40 km per year to reach Tuscany), but several hypotheses can be explored. First, the human-mediated movement of infested materials can play a key role in the dispersal of WTB, allowing the insect to cover hundreds or thousands of kilometers in a few days. This dispersal pathway was well documented in the USA (Newton and Fowler 2009; Jacobi et al. 2012). Another possibility is that P. juglandis was already present in Italy long before the year of discovery (2013) and it was found only when an active and specific survey had been implemented. This would have given the insect more time to disperse slowly into new areas and regions. Nevertheless, although certainly possible, this hypothesis does not explain how the presence of such aggressive species as WTB and TCD was not detected earlier. Lastly, reports of the presence of P. juglandis in various regions of Italy, even far from the first record, may be due to several independent introductions. This latter hypothesis seems extremely probable and could be tested by performing specific genetic analyses to compare specimens collected from populations sampled in the Italian regions where the pest has been recorded in the last years.
Pityophthorus juglandis represents only one of the insect alien species recently introduced in Europe that attack Juglans species. The walnut husk fly, Rhagoletis completa Cresson (Diptera: Tephritidae), is a pest originating from North America and introduced in Europe in the early 1990s (Duso and Dal Lago 2006; Verheggen et al. 2017). Megaplatypus mutatus (Chapuis) (Coleoptera: Curculionidae) is an ambrosia beetle native to South America (Wood 1993), accidentally introduced to Italy in 1998 (EPPO 2004), polyphagous on a wide range of forest and fruit tree species, including walnuts (Gonzalez-Audino et al. 2013). The black timber bark beetle, Xylosandrus germanus (Blandford) (Coleoptera: Curculionidae), is an ambrosia beetle native to Eastern Asia, Russian Far East and China introduced to both Europe (Groschke 1953) and USA, where is causing large damage to American walnut orchards (Weber and McPherson 1984). For the future protection of walnut orchards and to prevent further spreading of quarantine pests, phytosanitary measures for Juglans plants and their products are required (EPPO 2020) and specific and accurate monitoring of these environments have to be implemented.
Authors’ contribution
The authors contributed equally to data collection and analysis and to manuscript preparation.
Data availability
Raw data are available in the Supplementary Materials.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Audley JP, Bostock RM, Seybold SJ (2020a) Trap assays of the Walnut Twig Beetle, Pityophthorus juglandis Blackman (Coleoptera: Curculionidae: Scolytinae), reveal an effective semiochemical repellent combination. J Chem Ecol 46:1047–1058. https://doi.org/10.1007/s10886-020-01228-9
Audley JP, Dallara P, Nelson LJ, Hamud SM, Bostock RM, Seybold SJ (2020b) Trapping failure leads to discovery of potent semiochemical repellent for the Walnut Twig Beetle. J Econ Entomol 113(6):2772–2784. https://doi.org/10.1093/jee/toaa257
Barnouin T, Soldati F, Roques A, Faccoli M, Kirkendall LR, Mouttet R, Daubree JB, Noblecourt T (2020) Bark beetles and pinhole borers recently or newly introduced to France Coleoptera: Curculionidae, Scolytinae and Platypodinae. Zootaxa. https://doi.org/10.11646/zootaxa.4877.1.2
Bosio G, Cooke-McEwen C (2018) Insects collected from wood infested with Pityophthorus juglandis Blackman (Coleoptera Curculionidae Scolytinae) in the Piemonte region, Northwestern Italy. Boll della Soc Entomol Ital 150:21–30. https://doi.org/10.4081/BollettinoSEI.2018.21
Bright, DEJ, Stark RW (1973) The bark and ambrosia beetles of California (Coleoptera: Scolytidae and Platypodidae). Bull Calif Insect Surv 16
Carrillo D, Duncan RE, Ploetz JN, Campbell F, Ploetz RC, Peña JE (2014) Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol 63:54–62. https://doi.org/10.1111/ppa.12073
Chen Y, Aukema BH, Seybold SJ (2020) The effects of weather on the flight of an invasive bark beetle. Pityophthorus juglandis Insects 11:156. https://doi.org/10.3390/insects11030156
Cranshaw W (2011) Recently recognized range extensions of the Walnut Twig Beetle, Pityophthorus juglandis Blackman (Coleoptera: Curculionidae: Scolytinae), in the Western United States. Coleopt Bull 65:48–49. https://doi.org/10.1649/0010-065X-65.1.48
Cranshaw W, Tisserat N (2012) Questions and answers about thousand cankers disease of walnut. Color State Univ Fort Collins, CO
de Rigo D, Enescu CM, Houston Durrant T, Tinner W, Caudullo G (2016) Juglans regia in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, et al. (eds) European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg
Duso C, Dal Lago G (2006) Life cycle, phenology and economic importance of the walnut husk fly Rhagoletis completa Cresson (Diptera: Tephritidae) in northern Italy. Ann la Société Entomol Fr 42:245–254. https://doi.org/10.1080/00379271.2006.10700628
Eichhorn MP, Paris P, Herzog F, Incoll LD, Liagre F, Mantzanas K, Mayus M, Moreno G, Papanastasis VP, Pilbeam DJ, Pisanelli A, Dupraz C (2006) Silvoarable systems in Europe—past, present and future prospects. Agrofor Syst 67:29–50. https://doi.org/10.1007/s10457-005-1111-7
European and Mediterranean Plant Protection Organization (EPPO) (2004) EPPO Reporting Service no.4
European and Mediterranean Plant Protection Organization (EPPO) (2015) Pest risk analysis for Thousand Cankers Disease (Geosmithia morbida and Pityophthorus juglandis). EPPO, Paris
European and Mediterranean Plant Protection Organization (EPPO) (2019) EPPO Reporting Service no.5 - Disease
European and Mediterranean Plant Protection Organization (EPPO) (2020) PM 8/12 (1) Juglans. EPPO Bulletin 50:107–119
European and Mediterranean Plant Protection Organization (EPPO) (2021) Pityophthorus juglandis. EPPO datasheets on pests recommended for regulation. Available online. https://gd.eppo.int
Eyre D, Haack RA (2017) Invasive cerambycid pests and biosecurity measures. In: Wang Q (ed) Cerambycidae of the World: Biology and Pest Management. CRC Press, Boca Raton, FL, pp 563–618
Faccoli M, Simonato M, Rassati D (2016) Life history and geographical distribution of the Walnut Twig Beetle, Pityophthorus juglandis (Coleoptera: Scolytinae), in southern Europe. J Appl Entomol 140:697–705. https://doi.org/10.1111/jen.12299
Favaro R, Wichmann L, Ravn HP, Faccoli M (2015) Spatial spread and infestation risk assessment in the Asian longhorned beetle, Anoplophora glabripennis. Entomol Exp Appl 155:95–101. https://doi.org/10.1111/eea.12292
Gonzalez-Audino P, Griffo R, Gatti P, Allegro G, Zerba E (2013) Pheromone detection of the introduced forest pest Megaplatypus mutatus (=Platypus mutatus) (Chapuis) (Platypodinae, Curculionidae) in Italy. Agrofor Syst 87:109–115. https://doi.org/10.1007/s10457-012-9527-3
Groschke F (1953) Der “schwarze nutzholzborkenkäfer”, Xylosandrus germanus Blandf., ein neuer schädling in Deutschland. Z Angew Entomol 34:297–302. https://doi.org/10.1111/j.1439-0418.1953.tb00698.x
Jacobi WR, Hardin JG, Goodrich BA, Cleaver CM (2012) Retail firewood can transport live tree pests. J Econ Entomol 105:1645–1658. https://doi.org/10.1603/EC12069
Kausrud KL, Grégoire J-C, Skarpaas O, Erbilgin N, Gilbert M, Økland B, Stenseth NC (2011) Trees wanted—dead or alive! Host selection and population dynamics in tree-killing bark beetles. PLoS ONE 6:e18274. https://doi.org/10.1371/journal.pone.0018274
Kees AM, Hefty AR, Venette RC, Seybold SJ, Aukema BH (2017) Flight capacity of the Walnut Twig Beetle (Coleoptera: Scolytidae) on a laboratory flight mill. Environ Entomol 46:633–641. https://doi.org/10.1093/ee/nvx055
Kirisits T (2007) Fungal associates of european bark beetles with special emphasis on the ophiostomatoid fungi. Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Springer, Netherlands, pp 181–236
Kirkendall L, Faccoli M (2010) Bark beetles and pinhole borers (Curculionidae, Scolytinae, Platypodinae) alien to Europe. Zookeys 56:227–251. https://doi.org/10.3897/zookeys.56.529
Kolařík M, Freeland E, Utley C, Tisserat N (2011) Geosmithia morbida sp. nov., a new phytopathogenic species living in symbiosis with the walnut twig beetle (Pityophthorus juglandis) on Juglans in USA. Mycologia 103:325–332. https://doi.org/10.3852/10-124
Leslie CA, Seybold SJ, Graves AD, Cranshaw W, Tisserat N (2009) Potential impacts of thousand cankers disease on commercial walnut production and walnut germplasm conservation. VI International Walnut Symposium 861:431–434
Malacrinò A, Rassati D, Schena L, Mehzabin R, Battisti A, Palmeri V (2017) Fungal communities associated with bark and ambrosia beetles trapped at international harbours. Fungal Ecol 28:44–52. https://doi.org/10.1016/j.funeco.2017.04.007
McCullagh P, Nelder J (1989) Generalized Linear Models, 2nd editio. Chapman and Hall, London, UK
Meurisse N, Rassati D, Hurley BP, Brockerhoff EG, Haack RA (2019) Common pathways by which non-native forest insects move internationally and domestically. J Pest Sci 92:13–27. https://doi.org/10.1007/s10340-018-0990-0
Montecchio L, Faccoli M (2014) First record of thousand cankers disease Geosmithia morbida and Walnut Twig Beetle Pityophthorus juglandis on Juglans nigra in Europe. Plant Dis 98:696–696. https://doi.org/10.1094/PDIS-10-13-1027-PDN
Montecchio L, Fanchin G, Simonato M, Faccoli M (2014) First record of thousand cankers disease fungal pathogen Geosmithia morbida and walnut twig beetle Pityophthorus juglandis on Juglans regia in Europe. Plant Dis 98:1445. https://doi.org/10.1094/PDIS-07-14-0719-PDN
Montecchio L, Vettorazzo M, Faccoli M (2016) Thousand cankers disease in Europe: an overview. EPPO Bull 46:335–340. https://doi.org/10.1111/epp.12301
Moricca S, Bracalini M, Benigno A, Ginetti B, Pelleri F, Panzavolta T (2019) Thousand cankers disease caused by Geosmithia morbida and its insect vector Pityophthorus juglandis first reported on Juglans nigra in Tuscany, Central Italy. Plant Dis 103:369–369. https://doi.org/10.1094/PDIS-07-18-1256-PDN
Newton L, Fowler G (2009) Pathway Assessment: Geosmithia sp. and Pityophthorus juglandis Blackman movement from the western into the eastern United States. U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Washington, D.C.
Nilssen AC (1984) Long-range aerial dispersal of bark beetles and bark weevils (Coleoptera, Scolytidae and Curculionidae) in northern Finland. Ann Entomol Fenn 50:37–42
Ploetz RC, Hulcr J, Wingfield MJ, de Beer ZW (2013) Destructive tree diseases associated with ambrosia and bark beetles: black swan events in tree pathology? Plant Dis 97:856–872. https://doi.org/10.1094/PDIS-01-13-0056-FE
R Core Team (2019) R version 3.6.1: A language and environment for statistical computing. R Found Stat Comput Vienna, Austria URL https//www R-project org
Regione del Veneto (2014a) Decreto del dirigente del settore servizi fitosanitari n. 30 del 14 agosto 2014. Misure fitosanitarie di controllo ed eradicazione di Geosmithia morbida in Regione Veneto. Bur n. 83 del 26/08/2014
Regione del Veneto (2014b) Decreto n. 43 del 6 novembre 2014. Misure fitosanitarie di controllo di Geosmithia morbida in Regione Veneto. Aggiornamento della zona delimitata
Regione del Veneto (2015) Decreto n. 08 del 6 Febbraio 2015. Misure fitosanitarie di controllo di Geosmithia morbida in Regione Veneto. Aggiornamento della zona delimitata
Rugman-Jones PF, Seybold SJ, Graves AD, Stouthamer R (2015) Phylogeography of the walnut twig beetle, Pityophthorus juglandis, the vector of thousand cankers disease in north american walnut trees. PLoS ONE 10:e0118264. https://doi.org/10.1371/journal.pone.0118264
Seebens H, Blackburn TM, Dyer EE et al (2018) No saturation in the accumulation of alien species worldwide. Nat Commun 8:E2264–E2273. https://doi.org/10.1073/pnas.1719429115
Seybold SJ, Coleman TW, Dallara PL, Dart NL, Graves AD, Pederson LA, Spichiger SE (2012) Recent collecting reveals new state records and geographic extremes in the distribution of the walnut twig beetle, Pityophthorus juglandis blackman (Coleoptera: Scolytidae), in the United States. Pan-Pac Entomol 88:277–280. https://doi.org/10.3956/2012-32.1
Seybold SJ, Haugen D, O’Brien J, Graves AD (2013) Thousand cankers disease. USDA Forest Service, Northeastern Area State and Private Forestry Pest Alert. NA-PR-02–10, originally published May 2010, reprinted Aug. 2010, Oct. 2010, and Feb. 2013. Available: http://www.na.fs.fed.us/pubs/detail.cfm, 2013.
Seybold SJ, Klingeman WE, Hishinuma SM, Coleman TW, Graves AD (2019) Status and impact of walnut twig beetle in urban forest, orchard, and native forest ecosystems. J For 117:152–163. https://doi.org/10.1093/jofore/fvy081
Six DL (2012) Ecological and evolutionary determinants of bark beetle —fungus symbioses. Insects 3:339–366. https://doi.org/10.3390/insects3010339
Tisserat N, Cranshaw W, Leatherman D, Utley C, Alexander K (2009) Black walnut mortality in Colorado caused by the walnut twig beetle and thousand cankers disease. Plant Heal Prog 10:10. https://doi.org/10.1094/PHP-2009-0811-01-RS
Verheggen F, Verhaeghe A, Giordanengo P, Tassus X, Escobar-Gutiérrez A (2017) Walnut husk fly, Rhagoletis completa (Diptera: Tephritidae), invades Europe: invasion potential and control strategies. Appl Entomol Zool 52:1–7. https://doi.org/10.1007/s13355-016-0459-7
Weber BC, McPherson JE (1984) Attack on black walnut trees by the ambrosia beetle Xylosandrus germanus (Coleoptera: Scolytidae). Forest Science 30:864–870. https://doi.org/10.1093/forestscience/30.4.864
Wiggins G, Grant J, Lambdin P, Merten P, Nix K, Hadziabdic D, Windham M (2014) Discovery of Walnut Twig Beetle, Pityophthorus juglandis, associated with forested black walnut, Juglans nigra, in the Eastern U.S. Forests 5:1185–1193. https://doi.org/10.3390/f5061185
Wilstermann A, Hoppe B, Schrader G, Delbianco A, Vos S (2020) Pest survey card on Geosmithia morbida and its vector Pityophthorus juglandis. EFSA Support Publ. https://doi.org/10.2903/sp.efsa.2020.EN-1894
Wood DL (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu Rev Entomol 27:411–446. https://doi.org/10.1146/annurev.en.27.010182.002211
Wood SL (1993) Revision of the genera of Platypodidae (Coleoptera). Great Basin Nat 53:259–281
Wood SL, Bright DE (1992) A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: taxonomic index. Gt Basin Natur Mem 13:1–1553
Acknowledgements
We thank Iris Bernardinelli, Giovanni Zanini, Marco Vettorazzo, Tiziano Visigalli, Giovanni Bosio, Dino Calzavara, Giovanni Raffaelli, Riccardo Favaro and Lars Wichmann for providing information and for their help during the field and laboratory work. We especially thank Alison Garside for proofreading the manuscript, the editor and four anonymous reviewers for their useful comments.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. This research was partially financed by the Regional Plant Protection Organization of the Veneto Region and by the DOR projects of the University of Padua.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or vertebrates performed by any of the authors.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marchioro, M., Faccoli, M. Dispersal and colonization risk of the Walnut Twig Beetle, Pityophthorus juglandis, in southern Europe. J Pest Sci 95, 303–313 (2022). https://doi.org/10.1007/s10340-021-01372-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01372-5