Abstract
Pierce-sucking insects, such as plant bugs or stink bugs, cause damage in cotton fields worldwide. A novel genetically engineered (GE) cotton (Gossypium hirsutum) is protected against hemipteran pests and thrips by producing the modified Bacillus thuringiensis (Bt) mCry51Aa2 protein. Herbivores that consume insect-protected GE crops, and their natural enemies, can be exposed to plant-produced insecticidal proteins. We investigated tritrophic interactions to evaluate the potential impact of the novel Bt cotton on a non-target herbivore, the spider mite Tetranychus urticae, and on a generalist predator, the pirate bug Orius majusculus. Pirate bugs belong to the same insect order as the target pests and might thus be adversely affected by mCry51Aa2. Enzyme-linked immunosorbent assays showed that levels of mCry51Aa2 in T. urticae were one order of magnitude, and those in O. majusculus were three orders of magnitude lower than in Bt cotton leaves. O. majusculus nymphs fed with spider mites collected from Bt cotton had lower survival, increased developmental time, and reduced fecundity compared to nymphs fed spider mites from non-Bt near-isogenic cotton. Because Bt cotton did not affect the survival and growth of the spider mites, we conclude that indirect prey-quality mediated effects of the Bt cotton on the predatory bugs are unlikely and that O. majusculus are directly affected by the Bt protein. Follow-up studies are indicated to assess whether the effects observed under worst-case conditions are mitigated under more realistic exposure conditions where alternative prey with low Bt protein levels is available.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Key message
-
Genetically engineered cotton producing a modified protein from the bacterium Bacillus thuringiensis is protected against plant bugs and thrips.
-
In laboratory experiments, Bt cotton was offered to beneficial minute pirate bugs in a plant–spider mite–predator food chain.
-
Spider mites containing high amounts of Bt protein caused increased mortality, delayed development, and reduced fecundity in the pirate bugs, when fed exclusively from nymph to adult.
-
Experiments with more realistic feeding scenarios are indicated to assess the ecological relevance of these findings.
Introduction
Since the commercial introduction of genetically engineered (GE) crops more than 20 years ago, the global cultivated area has increased to 192 million hectares in 2018 (ISAAA 2018). The majority of today’s insect-protected GE crops produce Cry proteins from the bacterium Bacillus thuringiensis (Bt) that specifically target certain lepidopteran or coleopteran pests. Worldwide, 80% of the cultivated cotton, Gossypium hirsutum L., carries GE traits, such as insect protection and/or herbicide tolerance (ISAAA 2018).
Cotton with protection against lepidopteran pests is among the most important GE crops and the plant-produced Cry proteins allowed for reduced applications of broad spectrum insecticides against damaging caterpillars (Klümper and Qaim 2014). While Bt cotton is highly efficient against the major lepidopteran pests, beneficial arthropods and non-lepidopteran herbivores remain unaffected. When broad spectrum insecticides are replaced by highly specific Bt plants, natural enemies become important to keep populations of secondary pests below thresholds for economic injury (Naranjo et al. 2015; Anderson et al. 2019).
In some Bt cotton systems, however, plant bugs (Hemiptera, suborder Heteroptera) are serious pests (Lu et al. 2010; Naranjo 2011). GE cotton event MON 88702 has been developed to target certain plant bugs (Gowda et al. 2016). The Bt protein Cry51Aa2 showed activity against Lygus spp. (Hemiptera: Miridae), but activity in transgenic cotton plants was not sufficient for effective protection. Therefore, MON 88702 containing a modified version of the protein was developed with more than 200-fold increased lethality to Lygus spp. compared to the unmodified Cry51Aa2 (Gowda et al. 2016). MON 88702 has been shown to effectively control Lygus spp. and also thrips (Frankliniella spp.; Thysanoptera: Thripidae) under field conditions (Akbar et al. 2019).
Before GE crops can be cultivated, they have to pass a risk assessment to evaluate potential effects on the ecosystem including biological control provided by natural enemies (Romeis et al. 2008). This is particularly pertinent in the case of cotton producing mCry51Aa2 since some natural enemies are in the same order (Hemiptera) as the target pests. Natural enemies could be adversely affected by ingested Bt proteins, for example when consuming prey containing Bt protein, or plant material, such as sap or pollen, to supplement their diet. In addition, indirect, prey-quality mediated effects can occur when predators or parasitoids feed on prey or hosts that are affected by the Bt proteins (i.e. “sick” prey/hosts) and thus provide a lower nutritional value for their natural enemies (Romeis et al. 2006; Naranjo 2011; Romeis et al. 2019). Early in the risk assessment process, the assessment of adverse effects on natural enemies is typically based on laboratory studies with purified Bt protein in artificial diets (first-tier studies) (Romeis et al. 2008). Such studies have the advantage to test high doses of the toxins provided in artificial diet or GE plant tissue under highly controlled laboratory conditions to enhance the ability to detect toxic effects. Higher tier assessments, including more complex laboratory scenarios, greenhouse studies, and field experiments, are then conducted as deemed necessary based on the outcome of the early tier studies or when required specifically by regulatory agencies (Romeis et al. 2008). In those studies, more realistic exposure scenarios can be simulated to evaluate whether previously identified hazards translate to potential risks in the field. Species to focus on the risk assessment should be representatives of valued taxa in the field that provide important ecological functions. In addition, they should be exposed to the produced insecticidal proteins, most likely sensitive, and available and amenable for testing (Romeis et al. 2013).
Laboratory studies reported that the purified Bt protein mCry51Aa2 was harmful to some non-target herbivores and natural enemies (Bachman et al. 2017). Three mirid bug species, one thrips species, and two chrysomelid beetle species were adversely affected by mCry51Aa2. In addition to those pest species, the predatory bug Orius insidiosus (Say) (Hemiptera: Anthocoridae) also showed susceptibility to the Bt protein. In contrast, no activity was seen for two coccinellid beetles, four lepidopteran species, one hymenopteran parasitoid, the honey bee, one collembolan, and one earthworm. The potential hazard for O. insidiosus identified by Bachman et al. (2017) indicates further studies with more realistic exposure scenarios including tritrophic experiments.
Laboratory experiments with previously commercialized Bt cotton varieties showed that caterpillars (Spodoptera spp.) with low susceptibility to the Bt proteins produced in Bt cotton as well as spider mites feeding on cotton contained relatively high levels of Cry1Ac and Cry2Ab (Eisenring et al. 2017; Meissle and Romeis 2018). Spider mites even contained higher concentrations of Cry1Ac than the leaf tissue in some studies (Torres and Ruberson 2008; Esteves Filho et al. 2010). In contrast, low or non-detectable levels of Bt proteins in aphids and other species feeding on phloem sap were reported (Romeis and Meissle 2011; Meissle and Romeis 2018). In general, Bt protein concentrations have been found to decrease from one trophic level to the next and most predators, including Orius spp., contained relatively low concentrations of Bt protein in the field (Meissle and Romeis 2009a; Eisenring et al. 2017; Romeis et al. 2019).
As a follow-up to the artificial diet study (Bachman et al. 2017), we worked in a tritrophic system that represents a more realistic worst-case exposure scenario for Orius spp. in Bt cotton with the spider mite Tetranychus urticae Koch (Acari: Tetranychidae) as prey. As discussed above, spider mites are appropriate for use as prey since they are important pests in many crops including cotton and they contain relatively high concentrations of Bt protein when feeding on Bt cotton while not being a target of the novel mCry51Aa2-producing cotton. In addition, they can be easily reared under laboratory conditions and natural enemies, such as Orius species, readily consume them.
In the present study, we focus on Orius predatory bugs since the genus Orius is one of the most dominant predator groups in cotton fields (Cisneros and Rosenheim 1998; Ellington et al. 1997; Eisenring et al. 2017). We selected Orius majusculus (Reuter) (Hemiptera: Anthocoridae), which is distributed all over Europe. We worked with a European species (instead of O. insidiosus, which is a new world species), mainly because of practical aspects, but the lifestyle is very similar to other widespread Orius species.
The tritrophic interactions among Bt cotton, T. urticae, and O. majusculus were tested in response to exposure to the mCry51Aa2 protein. The following research questions were addressed: (1) is T. urticae affected by mCry51Aa2-producing cotton? Knowledge on the susceptibility of the prey species for the produced Bt protein is important to know whether prey-quality mediated effects on the predator are to be expected (Romeis et al. 2019). (2) Is O. majusculus affected when feeding exclusively on spider mite prey from mCry51Aa2-producing cotton? (3) To which concentrations of mCry51Aa2 are spider mites and O. majusculus exposed? The generated data are relevant for the non-target risk assessment of this novel GE cotton event.
Methods
Cultivation of cotton
GE cotton expressing the mCry51Aa2 protein from B. thuringiensis (event MON 88702) and the non-transgenic near isoline (DP393) were provided by Bayer Crop Science (St. Louis, USA). Seeds were planted in 1.3L pots using heat-treated soil (60 °C for > 24 h) to minimize soil-borne pests and diseases and incubated in a growth chamber at 25 °C, 70% relative humidity, and a 16:8 light to dark cycle. After approximately 3 weeks, plants were transplanted into 3L pots using untreated (not heated) soil; 15 g slow-release fertilizer was applied with the soil (Manna 3 M, Wilhelm Haug GmbH, Ammerbuch, Germany). Subsequently, 0.2–0.8 L of liquid NPK fertilizer was applied weekly (0.2%, Manna, Wilhelm Haug GmbH).
Spider mite colony
A starter population of mixed stages of T. urticae spider mites on bean leaves was provided by Syngenta Crop Protection Münchwilen AG (Stein, Switzerland). Colonies were established on Bt and non-Bt cotton and remained on the respective cotton type for the whole experimental period (approximately 1 year) without deliberate mixing among plant types. The colonies were maintained in glasshouse cabins at approximately 25 °C and 16:8 (L:D) photoperiod and were supplied with cotton plants in the flowering and boll-forming stage. Bt and non-Bt colonies were either kept in separated glasshouse cabins or together in one large cabin, albeit spatially separated. Consequently, exchange between the two colonies was not completely excluded, but minimized. We measured the concentration of mCry51Aa2 in spider mites reared on Bt and non-Bt plants to evaluate the rate of exchange between colonies (see below).
Predatory bug culture
Orius majusculus was purchased from Andermatt Biocontrol AG (Grossdietwil, Switzerland) and cultured at Agroscope since October 2016. Colonies were maintained in ventilated 1.3L transparent plastic cylinders. Pods of coco bean (Phaseolus vulgaris L.) were provided for oviposition, and water was supplied in glass tubes with cotton plugs. Frozen eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae, Biotop, Livron-sur-Drôme, France) were provided on the sticky section of yellow Postit papers as food. The culture was maintained in the glasshouse at approximately 22 °C and 16:8 (L:D) photoperiod.
Spider mite assay 1: Development and fecundity on Bt and non-Bt cotton
This experiment was conducted to investigate whether life-table parameters of spider mites feeding on leaf discs of Bt cotton were affected when compared to non-Bt cotton, with a focus on development and reproduction.
Leaf discs (2.5 cm diameter) were cut from the youngest fully developed leaves of 5–6-week-old plants (typically six fully developed leaves). Each disc was cut from a different plant (20 Bt and 20 non-Bt plants) and placed upside down in a customized transparent plastic dish (5 cm diameter, 1 cm high) covered with a ventilated lid. The plastic dishes were lined with wet cotton pads (Migros Budget, Migros, Switzerland) that provided moisture to the leaf and prevented spider mites from leaving the leaf disc [method adapted from Li and Romeis (2010) and Shu et al. (2018)]. One female spider mite raised on Bt or non-Bt plants was placed on each leaf disc of the respective plant type by using a fine paint brush. On the next day, females were removed and all eggs except one were removed or destroyed (day 0). Egg hatching, survival, larval/nymphal stage, and the gender of adults were recorded daily. Cotton pads were rewetted as necessary throughout the experiment. Spider mites were transferred to new dishes for the first time as soon as larvae hatched. Subsequently, leaf discs were changed every 3–4 days. During the immature phase, disc change was postponed to the next day if the spider mite was in the process of moulting (immobile). Data collection ended for males after they reached the adult stage. Newly emerged females were paired with one male either from the experiment or from the respective culture for 2 days. Then, males were removed. Eggs were counted daily and either removed or destroyed except on days when the disc was changed. The old discs with the eggs were incubated to determine egg fertility. After 5 days, the number of unhatched eggs was counted. The experiment ended when the last female died. The whole experiment was conducted three times consecutively.
Spider mite assay 2: Development and adult measures on Bt and non-Bt cotton
A second series of assays with spider mites was conducted as follows: (1) due to the rather high juvenile mortality observed in the first assay (Table 1), juvenile development was once more investigated with improved methodology that avoided handling of juveniles. (2) The second experiment ended once nymphs became adults, which allowed for data collection on additional measures of adults, such as weight and size. (3) To assess quality of spider mites in long-term cultures (approximately 1 year), we tested whether the origin of the mothers of the juveniles used in the assay (Bt or non-Bt culture) influenced their performance on Bt or non-Bt cotton.
Leaf discs of 6–8-week-old plants were cut from 25 Bt and 25 non-Bt plants and placed in transparent plastic dishes as described previously. One female spider mite raised on Bt or non-Bt plants was placed on each leaf disc of the respective plant type. On the next day, females were removed (day 0). After 3 days, two eggs per leaf disc were cut out using fine scissors: one egg was transferred to a Bt leaf disc and the other transferred to a non-Bt disc. This resulted in 25 replications of each combination of plant-type mother origin and plant-type food (100 eggs altogether). Subsequently, egg hatching, survival, and development were recorded until adults emerged. Leaf discs were not changed during juvenile development to minimize handling effects. Adults were sexed, frozen, and later measured (body length and width) using a binocular microscope fitted with a scaled ocular. The weight of individual females was recorded on a microbalance (Mettler-Toledo MX5, Greifensee, Switzerland). The weight of males was too low to be measured individually with sufficient precision. Therefore, groups of 3–5 males from the same treatment were pooled and weighed as a group. For statistical analysis, the calculated average weight per male was used. This experiment was also conducted three times consecutively.
Predator assay 1: Survival on prey from Bt and non-Bt cotton
We first established that O. majusculus can develop on spider mites as exclusive food. A preliminary assay demonstrated that neonates consumed on average six non-Bt spider mites per day, increasing to more than 50 for fifth instars. All stages of spider mites (including eggs) are consumed by the predator (Figure S1, Supplementary Online Material). The preliminary assay confirmed that O. majusculus can develop well with exclusive spider mite prey.
The purpose of the first predator assay was to test whether neonates differ in their development to adulthood when exclusively fed with spider mites from Bt cotton or non-Bt cotton. To obtain neonates, fresh bean pods were placed in the culture vessels for egg laying. After 24 h, the pods with newly laid eggs were incubated in a growth chamber (Panasonic MLR 352 H-PE, Labtech Services, Villmergen, Switzerland) at 25 °C, 70% RH, and 16:8-h photoperiod until neonates hatched. Similar to the spider mite assays, 5 cm plastic dishes with ventilated lids were lined with moist cotton pads. One Bt or non-Bt cotton leaf disc (diameter 38 mm) was placed in each dish, and one O. majusculus neonate (< 24 h after hatching) was introduced. Spider mites from Bt or non-Bt cotton were provided to the respective leaf discs. To ensure ad libitum feeding, the amount of spider mites was increased during the experiment from approximately 10 for neonates to at least 60 for fifth instar O. majusculus (see Fig. S1, Supplemental Online Material). Leaf discs, dishes, and cotton pads were changed every 3–4 days to ensure continuously high exposure of the spider mites to the Bt protein.
Survival and developmental stage of O. majusculus were recorded daily until the nymphs became adults or died. Moultings were determined by the presence of exuvia. The experiment ended with adult emergence. It was conducted twice with ten replicates and another two times with 20 replicates per plant treatment for a total of 60 replicates per treatment.
Predator assay 2: Fecundity on prey from Bt and non-Bt cotton
To be able to compare fecundity of O. majusculus on Bt versus non-Bt cotton, a second assay was conducted. Because of the high mortality in the Bt treatment of the previous predator assay, O. majusculus neonates, which were most susceptible to Bt proteins, were raised on E. kuehniella eggs for 5 days. Then, each nymph was weighed (Mettler-Toledo MX5) and placed either on a Bt or a non-Bt cotton leaf disc as described for the previous predator assay. Spider mites of the respective cotton plant type were provided ad libitum, and the leaf discs were changed every 3–4 days throughout the experiment. When nymphs became adults, the sex was determined and the body weight was measured. One female and one male were placed together on a larger leaf disc (diameter 54 mm) in a ventilated plastic dish (diameter 75 mm, height 23 mm) lined with a moist cotton pad. Males were removed once the first eggs were laid. If no eggs were laid after 4 days, the male was replaced. The replacement of males was repeated every 4 days until eggs were laid or the female died. During the oviposition period, leaf discs were replaced every 2 days and the old discs were kept separately and monitored for egg hatching. Numbers of fresh eggs on the new discs and hatched nymphs on the old discs were recorded daily until all females died. The experiment was conducted twice. The first series started with 15 individuals in each treatment. Because of a lower number of females in the Bt treatment, the second series started with 20 individuals on non-Bt and 30 individuals on Bt. Nymphs that died 1 or 2 days after the start of the experiment were assumed to be damaged by handling and excluded (4 non-Bt, 2 Bt).
Determination of Cry protein in leaves, spider mites, and predatory bugs
During each experimental repetition of both spider mite assays, samples of leaves (5 mm diameter discs) were taken directly from the plants and frozen for the determination of Cry protein concentrations. In addition, in the second and third repetition of the first spider mite assay, leaf discs were incubated under the conditions of the experiment and samples were taken 4 days later. In the second spider mite assay (all repetitions), additional samples were collected after 7 days of incubation. Those matured leaf samples in comparison with the fresh leaf samples allow an estimation of the stability of the Cry protein during the time period between leaf disc changes.
At the end of each predator assay 1 (repetition 3 and 4) and assay 2 (both repetitions), leaf and spider mite samples were collected from the spider mite culture. At each time period, samples were collected from five groups of Bt cotton plants. To get an idea of the vertical distribution of Cry protein, samples were taken from the lower third, the middle third, and the upper third of the plants. Spider mite samples consisted of ca. 5 mg, leaf samples of ca. 10 mg. Sampled plants in the spider mite culture were in the flowering or boll-forming stage, but not necessarily of uniform age across the sampling periods.
To estimate Cry protein concentrations in the predator, neonates of O. majusculus were first reared on E. kuehniella eggs for 5 days. Subsequently, they were transferred to Bt cotton leaf discs and spider mites from the Bt culture were provided ad libitum. When the nymphs reached the fifth instar (after approximately 4 days), they were fed one more day with spider mites and then five samples consisting of six nymphs each were collected for Cry protein analysis. This procedure was repeated three times.
We also sampled leaves of non-Bt plants throughout the year and spider mites from the non-Bt culture as described previously. Cry protein concentrations in all five collected non-Bt leaf samples were below the limit of detection (LOD) of 0.0015 µg/g fresh weight (FW). Concentrations in all five spider mite samples were below the LOD when the Bt and non-Bt cultures were in separate glasshouse cabins (< 0.0011 µg/g FW). When both cultures were in one large glasshouse cabin, Cry protein was detected in the three collected spider mite samples from the non-Bt culture, but concentrations were below 1% of those from the Bt culture (0.2–0.7 µg/g FW).
All samples were weighed (FW) and stored at − 80 °C. For the extraction of Cry protein, 1 × tris–borate buffer with 0.05% Tween20 was added at a ratio of at least 50:1 buffer (µL) to sample fresh weight (mg). After adding one 3 mm tungsten carbide ball, the tissues were macerated in a Qiagen TissueLyser II (Qiagen, Hombrechtikon, Switzerland) fitted with 24-tube adapters for microreaction tubes (Qiagen) at 30 Hz for 2 min. Macerated samples were centrifuged at 13,000×g for 5 min, and the supernatant was used either undiluted (O. majusculus samples and all non-Bt samples) or diluted with 1 × PBST + 0.5% BSA depending on the expected concentration in the samples (Bt cotton leaves 500–1000×, spider mites 200×). The mCry51Aa2 protein concentrations were subsequently measured with a sandwich enzyme-linked immunosorbent assay (ELISA). On each ELISA plate, 2 × 8 purified mCry51Aa2 standards between 0.08 and 10 ng/mL were included, together with six blanks (PBST + 0.5% BSA), two positive controls (Bt cotton leaf extract of known concentration), and two negative controls (non-Bt cotton leaf extract).
96-well transparent flat bottom plates (nunc, Thermo Scientific, Rochester, NY, USA) were coated with 100 µL mouse anti-Cry51Aa antibody in 50 mM carbonate–bicarbonate + 150 mM NaCl buffer (4 µg antibody/mL buffer). Next day, the plates were washed three times with PBST (wash buffer, also used for subsequent washing steps) and blocked with 200 µL PBST + 1% (w/v) bovine serum albumin (BSA) for 1–2 h at 37 °C. After 3 × washing, 100 µL samples, standards, and blanks were loaded and incubated for 50–70 min at 37 °C. The plates were 3 × washed, loaded with 100 µL detection antibody in PBST + 0.5% BSA [goat anti-Cry51Aa(IgG)-biotin, 0.1 µL antibody/mL buffer], and incubated for 50–70 min at 37 °C. Plates were washed 3×, reconstituted NeutrAvidin (enzyme conjugate, 100 units/mL, diluted 15,000 × with PBST + 0.5% BSA) was loaded, and another incubation step at 37 °C for 50–70 min followed. After another 3 × washing, 100 µL TMB substrate solution was loaded and the colour reaction was developed at room temperature for 9–11 min. 100 µL 6 M phosphoric acid was added to stop the colour reaction, and absorbance was read at 450 nm with an infinite F200 plate reader (Tecan, Männedorf, Switzerland). The concentrations of mCry51Aa2 in each sample were determined with the standard curves using regression analyses based on a hyperbola model. The LOD was calculated based on 3 × SD of the OD values of the blanks of each plate. At least, five blanks were loaded on each plate.
Data analysis
For both spider mite assays, the following parameters were analysed statistically: egg hatching rate (first generation), days from egg laying to hatching, juvenile survival from egg hatching to adult emergence, days from egg hatching to adult emergence, and gender of adult. For spider mite assay 1, we also analysed days from female emergence to death (female longevity), number of eggs laid from female emergence to death (total fecundity), number of eggs laid per day (total number of recorded eggs divided by female longevity), and egg hatching rate (second generation). To assess the egg hatching rate of the second generation (fertility), the proportion of unhatched eggs compared to the total number of incubated eggs was calculated for each female. For spider mite assay 2, we analysed male and female length, width, and weight. For both predator assays, statistical analyses were conducted for juvenile survival from the start of the experiment to emergence of adults. For predator assay 1, the development time of each nymphal stage was analysed separately, because of the high mortality in the Bt treatment. For predator assay 2, days from the start of the experiment to emergence of adults, gender, female weight, female longevity, and the percentage of fecund females, preoviposition time (days from emergence of female to first eggs), total fecundity, daily fecundity, and percentage of hatching eggs were analysed.
All data used for statistical analysis, tables, and figures for this publication can be found in the Supplementary Online Material (Supplementary Data Sheet 1). Data from the spider mite and predator performance assays were analysed with linear mixed effect models (LMER) or generalized linear mixed effect models (GLMER) using R statistical software (R version 3.5.1, The R Foundation for Statistical Computing, Vienna, Austria). For all categorical factors, contrasts were set to orthogonal. Time data (egg hatching time, juvenile development time, female longevity, preoviposition time) were analysed by GLMER with Poisson distribution from the lme4 package. Binomial data (gender, juvenile survival, egg hatching of the first generation, proportion of fecund females) were analysed by GLMER with binomial distribution (logit link function). Weight, length, width, and number of eggs were analysed by LMER. Egg hatching of the second generation was analysed by LMER after arcsin square root transformation. Effects of factors and interactions were determined from an ANOVA table with Type III sum of squares (car package). All GLMER and LMER models included “plant-type food” as a fixed factor and “experimental repetition” as a random factor.
Spider mite assay 2 used a full factorial model with the second fixed factor “plant-type mother origin” and the additional random factor “mother identity”.
In the first predator assay, survival analysis was conducted with tools from the survival package. A survival object was created (Surv function), Kaplan–Meier estimates for Bt and non-Bt cotton were calculated (survfit), and a log-rank test was applied to test for differences between both food plant types. A survival plot was created with ggsurvplot from the survminer package. The duration of individual developmental stages was analysed with GLMER.
Power analyses were performed to estimate detectable differences. Effect sizes (d) for time and fecundity data were based on two-sided t tests, true sample sizes, means and SDs of the non-Bt treatment, a power of 80%, α level of 0.05, and assuming equal sample sizes (package pwr). Using d, hypothetical second means were calculated from the mean of the non-Bt group. The detectable differences were then calculated from the percentage difference of the hypothetical means relative to the non-Bt means. For ratios (e.g. gender), effect size was similarly calculated based on a test of two proportions (for details of effect size calculations, see Shu et al. 2018).
Results
Spider mite assay 1: Development and fecundity on Bt and non-Bt cotton
Spider mites fed with Bt and non-Bt cotton leaves showed similar life-table parameters (Table 1). None of the measured parameters was significantly different between the two cotton treatments (p ≥ 0.1, Table 1). The estimates of detectable differences based on power analysis (t test) revealed that egg hatching time was the most sensitive parameter, where a 5% difference from the control treatment could be detected (low variation, high N). The least sensitive parameter (highest variation, low N) was total fecundity, with a statistically detectable difference of 52%.
Spider mite assay 2: Development and adult measures on Bt and non-Bt cotton
The second spider mite assay showed similar results as the first assay (Table 2). Once more, none of the measured parameters was significantly different between the two cotton treatments (p ≥ 0.1, Table 2).
Plant type, on which the mother of the tested spider mite was reared, was significant for male width (p = 0.03). A weak interaction of plant-type mother origin × plant-type food was present for juvenile survival (p = 0.02).
The most sensitive parameters (based on power analysis) were egg hatching time (2% detectable difference) and the length and width measurements (2–5%). The least sensitive parameter was gender (18%).
Predator assay 1: Survival on prey from Bt and non-Bt cotton
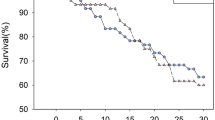
Survival of O. majusculus was reduced when fed prey from Bt compared to prey from non-Bt cotton (log-rank test, Chi2 = 44, p < 0.0001, Fig. 1a). In the non-Bt treatment, nymphs became adults between days 10 and 13 (Fig. 1b). In the Bt treatment, only six nymphs moulted into adults (days 13–16) (Fig. 1b). Developmental time in the Bt treatment was longer in all nymphal stages, but statistically different only for instars 1 and 3 (Table S1, Supplementary Online Material).
Survival of Orius majusculus when fed exclusively spider mites from Bt (MON 88702, red) or non-Bt cotton (DP393, blue). a Kaplan–Meier survival estimates with 95% confidence limits. Nymphs reaching adulthood are censored (marked with X). b Number of nymphs becoming adults (number of censored individuals) on each day of the experiment. Data are pooled from four experimental repetitions with a total N = 60
Predator assay 2: Fecundity on prey from Bt and non-Bt cotton
In contrast to the previous experiment with neonates, the 5-day-old nymphs survived well when fed with spider mites from Bt cotton (95%) (Table 3). Females were ca. 15% lighter when fed with prey from Bt cotton compared to prey from non-Bt cotton (p = 0.0001), and longevity was reduced by approximately one-third (p < 0.0001). Total and daily fecundity in the Bt treatment was only 20% and 30% of the non-Bt treatment, respectively (p < 0.0001). Body weight at the beginning of the experiment, juvenile development time, gender ratio, number of females that produced offspring, preoviposition time, and egg hatching rate were similar between Bt and non-Bt cotton (Table 3). Oviposition was highest at the beginning of the reproductive period and decreased continuously until death in both cotton treatments (Fig. 2).
Cry protein in leaves, spider mites, and predatory bugs
The mCry51Aa2 levels in matured leaves (at the time of replacement) collected during the spider mite assays differed from − 54% to + 12% compared to fresh leaves (Table 2, Supplementary Online Material). The mCry51Aa2 levels in leaves collected during the experiments with pirate bugs generally increased from the bottom third of the plant to the top third of the plant (Table S3, Supplementary Online Material). Measured concentrations in leaves ranged from 93 to 911 with a median of 207 µg/g FW (Fig. 3). The mCry51Aa2 levels in spider mites, which were sampled from the same plants, also increased from bottom to top (Table S3). Spider mites contained approximated one order of magnitude less mCry51Aa2 than leaves, ranging from 9.7 to 128 with a median of 29 µg/g FW (Fig. 3).
mCry51Aa2 concentrations in cotton leaves, spider mites (Tetranychus urticae), and predatory pirate bugs (Orius majusculus). Note that the y-axis is log scale. For more details of the individual samples, see Tables S3–S4. Samples were taken in parallel to the feeding assays with pirate bugs. Black dots represent individual values; red vertical lines represent medians
Finally, late O. majusculus nymphs that were kept on Bt cotton for 4 days contained two orders of magnitude lower concentrations than their prey with a median of 0.24 µg/g FW (Fig. 3, Table S4, Supplementary Online Material).
Discussion
Effects of Bt cotton on life history traits of herbivores and predators
In our study, Bt cotton producing mCry51Aa2 protein did not affect the survival, development, and growth of spider mites. Also, no effects were observed on spider mites after multiple generations feeding on Bt cotton, as demonstrated in the second spider mite assay, where eggs from mothers raised on Bt and non-Bt cotton were tested on both cotton types. Therefore, our findings indicate that T. urticae is not susceptible to plant-produced concentrations of mCry51Aa2 and indirect prey-quality mediated effects of the Bt cotton on the predatory bugs are unlikely (Romeis et al. 2019). These results are in line with those from previous studies demonstrating that spider mites are not sensitive to other Bt proteins, such as Cry1, Cry2, Cry3, or Cry34/35 (Dutton et al. 2002; Guo et al. 2016; Li and Romeis 2010; Lozzia et al. 2000; Shu et al. 2018).
Our study demonstrates that survival and development of first instar O. majusculus were negatively affected after feeding on Bt cotton-reared spider mites, while 5-day-old O. majusculus were not affected. Additionally, O. majusculus adults exhibited lower weight, shorter longevity, and decreased fecundity. Bachman et al. (2017) reported that survival of 5-day-old O. insidiosus was negatively affected after feeding on mCry51Aa2 protein incorporated in artificial diet (67% compared to 98% on diet without mCry51Aa2). Interestingly, development time to adulthood was not affected (10.9 ± 0.2 days on mCry51Aa2-containing diet and 10.5 ± 0.1 days on control diet) in that study. Several bi- and tritrophic studies have shown that Orius spp. were not affected by Bt maize producing Cry1Ab (Al-Deeb et al. 2001; Zwahlen et al. 2000) or Cry1F (Tian et al. 2014), Bt cotton producing Cry1Ac (Torres and Ruberson 2008) or Cry1Ac/Cry2Ab (Tian et al. 2014), purified Cry3Bb1 (Duan et al. 2008) or Cry34Ab1 + Cry35Ab1 (De Schrijver et al. 2016) mixed into artificial Orius-diet, or purified Cry1Ac, Cry1Ab or Cry2Ab provided in a tritrophic system (Gonzalez-Zamora et al. 2007). In contrast, Lumbierres et al. (2012) reported that fecundity and development time of O. majusculus were positively affected after feeding on Cry1Ab-expressing maize tissues (pollen and leaves) or on spider mites reared on Bt maize. They hypothesize that characteristics of the plant material other than the Cry1Ab were responsible for those effects.
In the present study, however, we worked with plants producing a modified Cry protein that is intended to reduce damage by heteropteran pests. In contrast to previous studies, susceptibility of predatory bugs can thus be hypothesized based on the target spectrum of mCry51Aa2 (Bachman et al. 2017).
mCry51Aa2 protein concentrations in Bt cotton and arthropods
During the course of the spider mite experiments, the mCry51Aa2 protein concentrations, and consequently exposure of spider mites and predators, remained relatively stable. mCry51Aa2 protein levels in cotton leaves generally decreased from young leaves at the top to old leaves at the bottom. In general, protein contents tend to be reduced as plants age (Sachs et al. 1998). Halfhill et al. (2003) demonstrated that the concentrations of Cry1Ac in the leaves of Bt oilseed rape (Brassica napus) decreased as leaves aged. In addition, field data show that older cotton plants expressing Cry1Ac and Cry2Ab2 contained less Bt protein than young plants (e.g. Greenplate 1999; Eisenring et al. 2017; Knight et al. 2013).
Spider mites are among the herbivores that contain the highest amounts of Bt protein when feeding on Bt crops. Meissle and Romeis (2018) showed that Cry2Ab concentrations in spider mites were approximately half of the concentrations measured in the cotton leaves, and Cry1Ac concentrations even exceeded the concentrations in leaves by a factor of 7. In addition, Torres and Ruberson (2008) have demonstrated that the Cry1Ac concentrations in spider mites exceeded the concentrations in the leaves by about 17 times and Esteves Filho et al. (2010) reported four times higher concentrations. These laboratory studies are supported by findings that most of Cry1Ab in Bt maize is present in mesophyll cells, which is the tissue that is mainly consumed by the spider mites (Dutton et al. 2004). In our study, mCry51Aa2 concentrations in spider mites were approximately one order of magnitude lower than in leaves. This demonstrates that expression patterns, digestion, and excretion dynamics vary among Bt proteins and plants. We believe that to date, our ELISA data are the first published with mCry51Aa2 in a herbivore, so comparison with other species is not possible. However, based on the literature available for other Bt proteins, we are confident that the concentrations measured in spider mites represent relatively high exposure to generalist predators (Romeis et al. 2019).
We also demonstrated that mCry51Aa2 protein is transmitted along the food chain to the predatory bug. Accordingly, the mCry51Aa2 concentration in O. majusculus represented approximately 1% of the concentration in the spider mite prey and thus 0.1% of the concentration in the leaves. Tian et al. (2014) found that adult O. insidiosus contained 3–4% of the Bt protein measured in Bt-resistant lepidopteran prey species (Trichoplusia ni and Spodoptera frugiperda) from laboratory-grown Bt plants (Cry2Ab-producing cotton, Cry1F-producing maize). In addition, Torres and Ruberson (2008) reported 17% of the Cry1Ac content of the western flower thrips on Bt cotton in O. insidiosus. In O. insidiosus collected in a Bt cotton field, Eisenring et al. (2017) detected the presence of Cry1Ac and Cry2Ab, while concentrations were approximately three orders of magnitude lower than in plant material. This confirms that Orius spp. do ingest Bt proteins in the cotton field, and the concentrations were similar to our tritrophic laboratory results.
For Bt maize producing Cry1Ab or Cry3Bb1, it was furthermore demonstrated that biological activity of the Bt proteins was maintained when T. urticae reared on Bt maize was fed to sensitive insects (Meissle and Romeis 2009b, Obrist et al. 2006). We thus assume that O. majusculus was also exposed to relatively high concentrations of bioactive mCry51Aa2 in the present study.
Conclusions
Our tritrophic bioassays with Bt cotton leaves, spider mites, and O. majusculus provide an experimental design for an early tier laboratory study that represents tritrophic worst-case exposure conditions through obligate feeding on spider mites. Based on the results of our study, O. majusculus can be reared on an exclusive diet of spider mites from neonate to adult, though this is unlikely to happen in a field scenario considering the generalist feeding behaviour of Orius spp. The continuous and high exposure to the mCry51Aa2 protein when feeding exclusively on spider mites that ingested Bt cotton led to lethal and sublethal effects on O. majusculus nymphs. While this indicates a potential hazard for pirate bugs, our results do not support a conclusion of risk in the field. Pirate bugs prey on many kinds of small arthropod species, and they can supplement their diet with plant materials, such as pollen (Dicke and Jarvis 1962; Kiman and Yeargan 1985). We selected spider mites as prey to ensure that O. majusculus ingest high amounts of Bt protein. In contrast, studies with other Bt crops have revealed that whiteflies and aphids contained no or at most traces of Bt protein (Romeis and Meissle 2011). Thus, pirate bugs in the field are likely to feed on a mix of different quality prey species with high, medium, and low mCry51Aa2 concentrations. The effects observed under worst-case tritrophic conditions might thus be mitigated when alternative, high-quality prey with low mCry51Aa2 levels is available. Follow-up studies including more realistic exposure conditions are currently being conducted to further understand the impact of mCry51Aa2-producing cotton on O. majusculus.
Authors’ contributions
MM, JR, and YJK conceived and designed the research. YJK and SK conducted experiments. MM and YJK analysed data. YJK and MM wrote the manuscript. All authors read and approved the manuscript.
References
Akbar W, Gowda A, Ahrens JE, Stelzer JW, Brown RS, Bollman SL, Greenplate JT, Gore J, Catchot AL, Lorenz G, Stewart SD, Kerns DL, Greene JK, Toews MD, Herbert DA, Reisig DD, Sword GA, Ellsworth PC, Godfrey LD, Clark TL (2019) First transgenic trait for control of plant bugs and thrips in cotton. Pest Manag Sci 75:867–877. https://doi.org/10.1002/ps.5234
Al-Deeb MA, Wilde GE, Higgins RA (2001) No effect of Bacillus thuringiensis corn and Bacillus thuringiensis on the predator Orius insidiosus (Hemiptera: Anthocoridae). Environ Entomol 30:625–629. https://doi.org/10.1603/0046-225X-30.3.625
Anderson JA, Ellsworth PC, Faria JC, Head GP, Owen MDK, Pilcher CD, Shelton AM, Meissle M (2019) Genetically engineered crops: importance of diversified integrated pest management for agricultural sustainability. Front Bioeng Biotechnol 7:24. https://doi.org/10.3389/fbioe.2019.00024
Bachman PM, Ahmad A, Ahrens JE, Akbar W, Baum JA, Brown S, Clark TL, Fridley JM, Gowda A, Greenplate JT, Jensen PD, Mueller GM, Odegaard ML, Tan J, Uffman JP, Levine SL (2017) Characterization of the activity spectrum of MON 88702 and the plant-incorporated protectant Cry51Aa2.834_16. PLoS ONE 12:e0169409. https://doi.org/10.1371/journal.pone.0169409
Cisneros JJ, Rosenheim JA (1998) Changes in the foraging behavior, within-plant vertical distribution, and microhabitat selection of a generalist insect predator: an age analysis. Environ Entomol 27:949–957. https://doi.org/10.1093/ee/27.4.949
De Schrijver A, Devos Y, De Clercq P, Gathmann A, Romeis J (2016) Quality of laboratory studies assessing effects of Bt-proteins on non-target organisms: minimal criteria for acceptability. Transgenic Res 25:395–411. https://doi.org/10.1007/s11248-016-9950-8
Dicke FF, Jarvis JL (1962) The habits and seasonal abundance of Orius insidiosus (Say) (Hemiptera-Heteroptera: Anthocoridae) on corn. J Kansas Entomol Soc 35:339–344
Duan JJ, Teixeira D, Huesing JE, Jiang C (2008) Assessing the risk to nontarget organisms from Bt corn resistant to corn rootworms (Coleoptera: Chrysomelidae): tier-I testing with Orius insidiosus (Heteroptera: Anthocoridae). Environ Entomol 37:838–844. https://doi.org/10.1603/0046-225X-30.3.625
Dutton A, Klein H, Romeis J, Bigler F (2002) Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol Entomol 27:441–447. https://doi.org/10.1046/j.1365-2311.2002.00436.x
Dutton A, Obrist L, D’Alessandro M, Diener L, Müller M, Romeis J, Bigler F (2004) Tracking Bt-toxin in transgenic maize to assess the risks on non-target arthropods. IOBC/WPRS Bull 27(3):57–63
Eisenring M, Romeis J, Naranjo SE, Meissle M (2017) Multitrophic Cry-protein flow in a dual-gene Bt-cotton field. Agric Ecosyst Environ 247:283–289. https://doi.org/10.1016/j.agee.2017.07.009
Ellington J, Southward M, Carrillo T (1997) Association among cotton arthropods. Environ Entomol 26:1004–1008. https://doi.org/10.1093/ee/26.5.1004
Esteves Filho AB, DeOliveira JV, Torres JT, Gondim MGC Jr (2010) Compared biology and behavior of Tetranychus urticae Koch (Acari: Tetranychidae) and Phytoseiulus macropilis (Banks) (Acari: Phytoseiidae) on Bollgard™ and non-transgenic isoline cotton. Neotrop Entomol 39:338–344. https://doi.org/10.1590/S1519-566X2010000300005
Gonzalez-Zamora JE, Camunez S, Avilla C (2007) Effects of Bacillus thuringiensis Cry toxins on developmental and reproductive characteristics of the predator Orius albidipennis (Hemiptera: Anthocoridae) under laboratory conditions. Environ Entomol 36:1246–1253. https://doi.org/10.1093/ee/36.5.1246
Greenplate JT (1999) Quantification of Bacillus thuringiensis insect control protein Cry1Ac over time in Bollgard cotton fruit and terminals. J Econ Entomol 92:1377–1383. https://doi.org/10.1093/jee/92.6.1377
Gowda A, Rydel TJ, Wollacott AM, Brown RS, Akbar W, Clark TL, Flasinski S, Nageotte JR, Read AC, Shi X, Werner BJ, Pleau MJ, Baum JA (2016) A transgenic approach for controlling Lygus in cotton. Nat Commun 7:12213. https://doi.org/10.1038/ncomms12213
Guo YY, Tian JC, Shi WP, Dong XH, Romeis J, Naranjo SE, Hellmich RL, Shelton AM (2016) The interaction of two-spotted spider mites, Tetranychus urticae Koch, with Cry protein production and predation by Amblyseius andersoni (Chant) in Cry1Ac/Cry2Ab cotton and Cry1F maize. Transgenic Res 25:33–44. https://doi.org/10.1007/s11248-015-9917-1
Halfhill MD, Millwood RJ, Rufty TW, Weissinger AK, Stewart CN Jr (2003) Spatial and temporal patterns of green fluorescent protein (GFP) fluorescence during leaf canopy development in transgenic oilseed rape, Brassica napus L. Plant Cell Rep 22:338–343. https://doi.org/10.1007/s00299-003-0696-4
ISAAA (2018) Global status of commercialized biotech/GM crops in 2018. ISAAA Brief 54
Kiman ZB, Yeargan KV (1985) Development and reproduction of the predator Orius insidiosus (Hemiptera: Anthocoridae) reared on diets of selected plant material and arthropod prey. Ann Entomol Soc Am 78:464–467. https://doi.org/10.1093/aesa/78.4.464
Klümper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS ONE 9(11):e111629. https://doi.org/10.1371/journal.pone.0111629
Knight K, Head G, Rogers J (2013) Season-long expression of Cry1Ac and Cry2Ab proteins in Bollgard II cotton in Australia. Crop Prot 44:50–58. https://doi.org/10.1016/j.cropro.2012.10.014
Li Y, Romeis J (2010) Bt maize expressing Cry3Bb1 does not harm the spider mite, Tetranychus urticae, or its ladybird beetle predator, Stethorus punctillum. Biol Control 53:337–344. https://doi.org/10.1016/j.biocontrol.2009.12.003
Lozzia GC, Rigamonti I, Manachini B, Rocchetti R (2000) Laboratory studies on the effects of transgenic corn on the spider mite Tetranychus urticae Koch. Boll Zool Agrar Bachic 32:35–47
Lu YH, Wu KM, Jiang YY, Xia B, Li P, Feng HQ, Wyckhuys KAG, Guo YY (2010) Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328:1151–1154. https://doi.org/10.1126/science.1187881
Lumbierres B, Albajes R, Pons X (2012) Positive effect of Cry1Ab-expressing Bt maize on the development and reproduction of the predator Orius majusculus under laboratory conditions. Biol Control 63:150–156. https://doi.org/10.1016/j.biocontrol.2012.07.009
Meissle M, Romeis J (2009a) The web-building spider Theridion impressum (Araneae: Theridiidae) is not adversely affected by Bt maize resistant to corn rootworms. Plant Biotechnol J 7:645–656. https://doi.org/10.1111/j.1467-7652.2009.00431.x
Meissle M, Romeis J (2009b) Insecticidal activity of Cry3Bb1 expressed in Bt maize on larvae of the Colorado potato beetle, Leptinotarsa decemlineata. Entomol Exp Appl 131:308–319. https://doi.org/10.1111/j.1570-7458.2009.00859.x
Meissle M, Romeis J (2018) Transfer of Cry1Ac and Cry2Ab proteins from genetically engineered Bt cotton to herbivores and predators. Insect Sci 25:823–832. https://doi.org/10.1111/1744-7917.12468
Naranjo SE (2011) Impacts of Bt transgenic cotton on integrated pest management. J Agric Food Chem 59:5842–5851. https://doi.org/10.1021/jf102939c
Naranjo SE, Ellsworth PC, Frisvold G (2015) Economic value of biological control in IPM of managed plant systems. Annu Rev Entomol 60:621–645. https://doi.org/10.1146/annurev-ento-010814-021005
Obrist LB, Dutton A, Romeis J, Bigler F (2006) Biological activity of Cry1Ab toxin expressed by Bt maize following ingestion by herbivorous arthropods and exposure of the predator Chrysoperla carnea. BioControl 51:31–48. https://doi.org/10.1007/s10526-005-2936-8
Romeis J, Meissle M (2011) Non-target risk assessment of Bt crops—Cry protein uptake by aphids. J Appl Entomol 135:1–6. https://doi.org/10.1111/j.1439-0418.2010.01546.x
Romeis J, Meissle M, Bigler F (2006) Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat Biotechnol 24:63–71. https://doi.org/10.1038/nbt1180
Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens MMC, Hartley SE, Hellmich RL, Huesing JE, Jepson PC, Layton R, Quemada H, Raybould A, Rose RI, Schiemann J, Sears MK, Shelton AM, Sweet J, Vaituzis Z, Wolt JD (2008) Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat Biotechnol 26:203–208. https://doi.org/10.1038/nbt1381
Romeis J, Raybould A, Bigler F, Candolfi MP, Hellmich RL, Huesing JE, Shelton AM (2013) Deriving criteria to select arthropod species for laboratory tests to assess the ecological risks from cultivating arthropod-resistant genetically engineered crops. Chemosphere 90:901–909. https://doi.org/10.1016/j.chemosphere.2012.09.035
Romeis J, Naranjo SE, Meissle M, Shelton AM (2019) Genetically engineered crops help support conservation biological control. Biol Control 130:136–154. https://doi.org/10.1016/j.biocontrol.2018.10.001
Sachs ES, Benedict JH, Stelly DM, Taylor JF, Altman DW, Berberich SA, Davis SK (1998) Expression and segregation of genes encoding Cry1A insecticidal proteins in cotton. Crop Sci 38:1–11. https://doi.org/10.2135/cropsci1998.0011183X003800010001x
Shu Y, Romeis J, Meissle M (2018) No interactions of stacked Bt maize with the non-target aphid Rhopalosiphum padi and the spider mite Tetranychus urticae. Front Plant Sci 9:39. https://doi.org/10.3389/fpls.2018.00039
Tian JC, Long LP, Wang XP, Naranjo SE, Romeis J, Hellmich RL, Wang P, Shelton AM (2014) Using resistant prey demonstrates that Bt plants producing Cry1Ac, Cry2Ab, and Cry1F have no negative effects on Geocoris punctipes and Orius insidiosus. Eviron Entomol 43:242–251. https://doi.org/10.1603/EN13184
Torres J, Ruberson JR (2008) Interactions of Bacillus thuringiensis Cry1Ac toxin in genetically engineered cotton with predatory heteropterans. Transgenic Res 17:345–354. https://doi.org/10.1007/s11248-007-9109-8
Zwahlen C, Nentwig W, Bigler F, Hillbeck A (2000) Tritrophic interactions of transgenic Bacillus thuringiensis corn, Anaphothrips obscurus (Thysanoptera: Thripidae), and the predator Orius majusculus (Heteroptera: Anthocoridae). Environ Entomol 29:846–850. https://doi.org/10.1603/0046-225X-29.4.846
Acknowledgements
Open access funding was provided by Agroscope. We would like to thank Oliver Kindler (Syngenta) for providing spider mites and Bayer Crop Science for providing cotton seeds as well as materials and protocols for ELISA measurements. We thank Christopher Brown and Lieselot Bertho for comments on an earlier draft of this manuscript. The study was supported by a Swiss Government Excellence Scholarship to Young-Joong Kim for the academic year 2017–2018 (ESKAS No. 2017.0260).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by I. Hiltpold.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, YJ., Kloos, S., Romeis, J. et al. Effects of mCry51Aa2-producing cotton on the non-target spider mite Tetranychus urticae and the predatory bug Orius majusculus. J Pest Sci 94, 351–362 (2021). https://doi.org/10.1007/s10340-020-01260-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-020-01260-4