Abstract
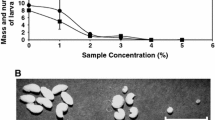
The outer-covering of seeds is known as the seed coat, and this is the seed's first protective barrier against the penetration of insects. In this sense, seed coat toxicity can affect insect development. In this work, we study the influence of the Canavalia ensiformis seed coat on embryonic and larval development of Callosobruchus maculatus. Oviposition of C. maculatus was negatively affected by the seed coat, while embryonic development occurred without any morphological or biochemical alterations. During larval development, physiological changes comprising delay in body weight, the inability of larvae to penetrate the C. ensiformis seed coat, and decreases in biochemical parameters, such as contents of glucose, proteins, and triglycerides, were recorded. Although larvae survived until 17 days after oviposition, they could not penetrate the C. ensiformis seed coat. A dose-dependent effect was observed on female oviposition, mass, and survival of larvae of C. maculatus. Cysteine protease, α-amylase and α-glucosidase activities decreased while aspartic protease activity increased with increases in ingestion of seed coat flour. Larval post-embryonic development was affected by C. ensiformis seed and this interference is related with a toxic seed coat which reduced activities of several key digestive enzymes, resulting in severe weight loss.







Similar content being viewed by others
References
Ahn JE, Zhu-Salzman K (2009) CmCatD, a cathepsin D-like protease has a potential role in insect defense against a phytocystatin. J Insect Physiol 55:678–685
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bridge PD, Sawilowsky SS (1999) Increasing physicians’ awareness of the impact of statistics on research outcomes: comparative power of the t-test and and Wilcoxon Rank-Sum test in small samples applied research. J Clin Epidemiol 52:229–235
Carlini CR, Oliveira AEA, Azambuja P, Xavier-Filho J, Wells MA (1997) Biological effects of canatoxin in different insect models: evidence for a proteolytic activation of the toxin by insect cathepsin like enzymes. J Econ Entomol 90:340–348
Chi YH, Salzman RA, Balfe S, Ahn JE, Sun W, Moon J, Yun DJ, Lee SY, Higgins TJ, Pittendrigh B, Murdock LL, Zhu-Salzman K (2009) Cowpea bruchid midgut transcriptome response to a soybean cystatin-costs and benefits of counter-defence. Insect Mol Biol 18:97–110
Cruz LP, De Sá LFR, Santos LA, Gravina GA, Carvalho AO, Fernandes KVS, Freire-Filho FR, Gomes VM, Oliveira AEA (2016) Evaluation of resistance in different cowpea cultivars to Callosobruchus maculatus infestation. J Pest Sci 89:117–128
De Sá LFR, Wermelinger TT, Ribeiro ES, Gravina GA, Fernandes KVS, Xavier-Filho J, Venancio TM, Rezende GL, Oliveira AEA (2014) Effects of Phaseolus vulgaris (Fabaceae) seed coat on the embryonic and larval development of the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). J Insect Physiol 60:50–57
Fabres A, Silva JCM, Fernandes KVS, Xavier-Filho J, Rezende GL, Oliveira AEA (2014) Comparative performance of the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae) on different plant diets. J Pest Sci 87:495–506
Hildebrandt A, Bickmeyer I, Kuhnlein RP (2011) Reliable drosophila body fat quantification by a coupled colorimetric assay. Plos One 6:1–6
Kingsolver JM (2004) Handbook of the Bruchidae of the United States and Canada, vol 1. United States Department of Agriculture (USDA), Washington, pp 82–85
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lemos FJA, Campos FAP, Silva CP, Xavier- Filho J (1990) Proteinases and amylases of larval midgut of Zabrotes subfasciatus reared on cowpea (Vigna unguiculata) seeds. Entomol Exp Appl 56:219–227
Liener IE (1976) Isolation and properties of concanavalin A. In: Bittiger H, Schnebli HP (eds) Concanavalin A as a Tool. Wiley, London, pp 17–31
Michaud D, Nguyen-Quoc B, Bernier-Vadnais N, Faye L, Yelle S (1994) Cysteine proteinase forms in sprouting potato tuber. Plant Physiol 90:497–503
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Monteiro RQ, Dutra DLS, Machado OLT, Carlini CR, Guimarães JA, Bom C, Zingali RB (1998) Bothrops jararaca snakes produce several bothrojaracin isoforms following an individual pattern. Comp Biochem Physiol 120:791–798
Naseri B, Fathipour Y, Moharramipour S, Hosseininaveh V, Gatehouse AMR (2010) Digestive proteolytic and amylolytic activities of Helicoverpa armigera in response to feeding on different soybean cultivars. Pest Manag Sci 66:1316–1323
Nogueira FCS, Silva CP, Alexandre D, Samuels RI, Soares EL, Aragão FJ, Palmisano G, Domont GB, Roepstorff P, Campos FA (2012) Global proteome changes in larvae of Callosobruchus maculatus (Coleoptera: Chrysomelidae:Bruchinae) following ingestion of a cysteine proteinase inhibitor. Proteomics 00:1–12
Oliveira AEA, Sales MP, Machado OLT, Fernandes KVS, Xavier-Filho J (1999) The toxicity of Jack bean (Canavalia ensiformis) cotyledon and seed coat proteins to the cowpea weevil (Callosobruchus maculatus). Entomol Exp Appl 92:249–255
Oliveira AEA, Sassaki GL, Iacomini M, Cunha M, Fernandes KVS (2001) Isolation and characterization of galactorhamnan polysaccharide from the seed coat of Canavalia ensiformis that is toxic to the cowpea weevil Callosobruchus maculatus. Entomol Exp Appl 101:225–231
Pedra JHF, Brandt A, Westerman R, Lobo N, Li HM, Romero-Severson J, Murdock LL, Pittendrigh BR (2003) Transcriptome analysis of the cowpea weevil bruchid: identification of putative proteinases and alpha-amylases associated with food breakdown. Insect Mol Biol 12:405–412
Rosenthal GA (1991) The biochemical basis for the deleterious effects of L-canavanine. Phytochemistry 30:1055–1058
Shaaya E, Kostjukovski M, Eilberg J, Sukprakarn C (1997) Plant oils as fumigants and contact insecticides for the control of stored-product insects. J Sci Food Agric 33:7–15
Silva CP, Xavier-Filho J (1991) Comparison between the levels of aspartic and cysteine proteinases of the larval midguts of Callosobruchus maculatus (F.) and Zabrotes subfasciatus (BOH.) (Coleoptera: bruchidae). Comp Biochem Physiol 99:529–533
Silva CP, Terra W, Ribeiro Xavier-Filho J, Sá MFG, Lopes AR, Pontes EG (1999) Digestion in larvae of Callosobruchus maculatus and Zabrotes subfasciatus (Coleoptera: Bruchidae) with emphasis on α-amylases and oligosaccharidases. Insect Biochem Mol Biol 29:355–366
Smith PK, Krohn GT, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 1:76–85
Souza SM, Uchôa AF, Silva JR, Samuels RI, Oliveira AEA, Oliveira EM, Linhares RT, Alexandre D, Silva CP (2010) The fate of vicilins, 7S storage globulins, in larvae and adult Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae). J Insect Physiol 56:1130–1138
Souza AJ, Santos PO, Pinto MST, Wermelinger TT, Ribeiro ES, Souza SC, Deus MF, Souza MC, Xavier-Filho J, Fernandes KVS, Oliveira AEA (2011) Natural seed coats provide protection against penetration by Callosobruchus maculatus (Coleoptera: Bruchidae) larvae. Crop Prot 30:651–657
Staneva E (1982) A study of bean seed beetle Callosobruchus maculatus F. (Coleoptera, Bruchidae) nutrient plants. Plant Sci 4:111–119
Terra WR, Ferreira C, De Bianchi AG (1979) Distribution of digestive enzymes among the endo and ectoperitrophic spaces and midgut cells of Rhynchosciara and its physiological significance. J Insect Physiol 25:423–434
Acknowledgements
This work was supported by grants from the Brazilian agencies: FAPERJ, CNPq and from the Universidade Estadual do Norte Fluminense Darcy Ribeiro-UENF.
Funding
The authors thank financial support for this work from FAPERJ (Fundação de amparo a pesquisa do Rio de Janeiro), CNPq (Conselho Nacional de Desenvolvimento Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). We would like to thank Dr Gustavo Lazzaro Rezende (LQFPP/CBB/UENF) for the contributions in the experiments of the insect embryonic morphology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by C. G. Athanassiou.
Rights and permissions
About this article
Cite this article
de Sá, L.F.R., Ventury, K.E., Machado, O.L.T. et al. Toxic effect of Canavalia ensiformis seed coat on larval development of Callosobruchus maculatus . J Pest Sci 91, 313–326 (2018). https://doi.org/10.1007/s10340-017-0895-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0895-3