Abstract
In this article, we describe and compare two individual-based models constructed to investigate how genetic factors influence the development of phosphine resistance in lesser grain borer (R. dominica). One model is based on the simplifying assumption that resistance is conferred by alleles at a single locus, while the other is based on the more realistic assumption that resistance is conferred by alleles at two separate loci. We simulated the population dynamic of R. dominica in the absence of phosphine fumigation, and under high and low dose phosphine treatments, and found important differences between the predictions of the two models in all three cases. In the absence of fumigation, starting from the same initial frequencies of genotypes, the two models tended to different stable frequencies, although both reached Hardy–Weinberg equilibrium. The one-locus model exaggerated the equilibrium proportion of strongly resistant beetles by 3.6 times, compared to the aggregated predictions of the two-locus model. Under a low dose treatment the one-locus model overestimated the proportion of strongly resistant individuals within the population and underestimated the total population numbers compared to the two-locus model. These results show the importance of basing resistance evolution models on realistic genetics and that using oversimplified one-locus models to develop pest control strategies runs the risk of not correctly identifying tactics to minimise the incidence of pest infestation.










Similar content being viewed by others
References
Andrewartha HG, Birch LC (1982) Selections from “the distribution and abundance of animals”. The University of Chicago
Arbogast RT (1991) Beetles: Coleoptera. In: Gorham JR (ed) Ecology and management of food-industry pests. Association of Official Analytical Chemists, Arlington, pp 131–176
Baldassari N, Martini A, Cavicchi S, Baronio P (2005) Effects of low temperatures on adult survival and reproduction of Rhyzopertha dominica. Bull Insectol 58(2):131–134
Banks HJ (1989) Behaviour of gases in grain storages. In: Fumigation and controlled atmosphere storage of grain. Proceedings of an international conference held at Singapore, pp 96–107
Beckett SJ, Longstaff BC, Evans DE (1994) A Comparison of the demography of four major stored grain Coleopteran pests and its implications for pest management. In: Proceedings of the 6th international conference on stored-product protection, CAB, Wallingford, Canberra, Australia, vol 1, pp 491–497
Birch LC (1945) The influence of temperature on the development of the different stages of Calandra oryzae L. and Rhizopertha dominica Fab. (Coleoptera). Aust J Exp Biol Med Sci 23(1):29–35
Birch LC (1948) The intrinsic rate of increase of an insect population. J Anim Ecol 17:15–26
Birch LC (1953) Experimental background to the study of the distribution and abundance of insects: I. The influence of temperature, moisture and food on the innate capacity for increase of three grain beetles. Ecology 34(5):698–711
Bunce NJ, Remillard RB (2003) Haber’s rule: the search for quantitative relationships in toxicology. Human Ecol Risk Assess 9(6):973–985
Collins PJ (1998) Resistance to grain protectants and fumigants in insect pests of stored products in Australia. In: Banks HJ et al. (eds) Proceedings of the first australian postharvest technical conference, Canberra, Australia, pp 55–57
Collins PJ, Daglish GJ, Nayak MK, Ebert PR, Schlipalius DI, Chen W, Pavic H, Lambkin TM, Kopittke RA, Bridgeman BW (2000) Combating resistance to phosphine in Australia. In: Proceedings of the international conference for controlled atmosphere and fumigation in stored products, Fresno CA, pp 593–607
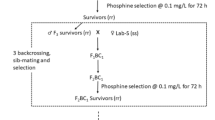
Collins PJ, Daglish GJ, Bengston M, Lambkin TM, Pavic H (2002) Genetics of resistance to phosphine in Rhyzopertha dominica (Coleoptera: Bostrichidae). J Econ Entomol 95(4):862–869
Collins PJ, Daglish GJ, Pavic H, Kopittke RA (2005) Response of mixed-age cultures of phosphine resistant and susceptible strains of lesser grain borer, Rhyzopertha dominica, to phosphine at a range of concentrations and exposure periods. J Stored Prod Res 41:373–385
Daglish GJ (2004) Effect of exposure period on degree of dominance of phosphine resistance in adults of Rhyzopertha dominica (Coleoptera: Bostrychidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag Sci 60(8):822–826
Driscoll R, Longstaff BC, Beckett S (2000) Prediction of insect populations in grain storage. J Stored Prod Res 36:131–151
FAO (1975) Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides: tentative method for adults of some major species of stored cereals with methyl bromide and phosphine. FAO Method No 16, FAO Plant Prot Bull 23:12–25
Gernert D (2007) Ockham’s razor and its improper use. J Sci Explor 21(1):135–140
Grimm V, Railsback SF (2004) Individual-based modeling and ecology. Princeton University Press, Princeton
Hagstrum DW, Flinn PW (1990) Simulations comparing insect species differences in response to wheat storage conditions and management practices. J Econ Entomol 83(6):2469–2475
Hagstrum DW, Subramanyam B (2009) Stored-product insect source. AACC International, St Paul
Herron GA (1990) Resistance to grain protectants and phosphine in coleopterous pests of grain stored on farms in New South Wales. Aust J Entomol 29:183–189
Lilford K (2009) Mathematical modelling of genetic resistance in stored-grain pests. Honours Thesis, Queensland University of Technology
Lilford K, Fulford G, Ridley A, Schlipalius DI (2009) Fumigation of stored grain insects a two locus model of phosphine resistance. In: 18th World IMACS/MODSIM Congress, Cairns, Australia
Limpert E, Stahel WA, Abbt M (2001) Log-normal distributions across the sciences: keys and clues: on the charms of statistics, and how mechanical models resembling gambling machines offer a link to a handy way to characterize log-normal distributions, which can provide deeper insight into variability and probability—normal or log-normal: that is the question. Bioscience 51(5):341–352
Longstaff BC (1999) An experimental and modelling study of the demographic performance of Rhyzopertha dominica (F) I: development rate. J Stored Prod Res 35:89–98
Matin ASM, Hooper GHS (1974) Susceptibility of Rhyzopertha dominica to ionizing radiation. J Stored Prod Res 10:199–207
Peck SL (2004) Simulation as experiment: a philosophical reassessment for biological modelling. Trends Ecol Evol 19(10):530–534
Peck SL (2008) The hermeneutics of ecological simulation. Biol Philos 23(3):383–402
Pimentel MAG, Faroni LA, Totola MR, Guedes RNC (2007) Phosphine resistance, respiration rate and fitness consequences in stored-product insects. Pest Manag Sci 63:876–881
Rees D (2004) Insects of stored products. CSIRO, Collingwood
Renton M (2009) The weeds fight back: Individual-based simulation of evolution of polygenic resistance to herbicides. In: Anderssen RS, Braddock RD, Newham LTH (eds) 18th world IMACS congress and MODSIM09 international congress on modelling and simulation. MSSANZ and IMACS, Cairns, Australia, pp 574–580
Renton M (2011) How much detail and accuracy is required in plant growth sub-models to address questions about optimal management strategies in agricultural systems? AoB Plants. doi:10.1093/aobpla/plr006
Renton M, Diggle A, Manalil S, Powles S (2011) Does cutting herbicide rates threaten the sustainability of weed management in cropping systems? J Theor Biol 283(1):14–27
Schlipalius DI, Cheng Q, Reilly PE, Collins PJ, Ebert PR (2002) Genetic linkage analysis of the lesser grain borer Rhyzopertha dominica identifies two loci that confer high-level resistance to the fumigant phosphine. Genetics 161:773–782
Schlipalius DI, Collins PJ, Mau Y, Ebert PR (2006) New tools for management of phosphine resistance. Outlooks Pest Manag 17:52–56
Schlipalius DI, Chen W, Collins PJ, Nguyen T, Reilly PEB, Ebert PR (2008) Gene interactions constrain the course of evolution of phosphine resistance in the lesser grain borer, Rhyzopertha dominica. Heredity 100:506–516
Schwardt HH (1933) Life history of the lesser grain borer. J Kansas Entomol Soc 6(2):61–66
Shi M, Renton M (2011) Numerical algorithms for estimation and calculation of parameters in modelling pest population dynamics and evolution of resistance in modelling pest population dynamics and evolution of resistance. Math Biosci 233(2):77–89
Shi M, Renton M, Collins PJ (2011) Mortality estimation for individual-based simulations of phosphine resistance in lesser grain borer (Rhyzopertha dominica). In: Chan F, Marinova D, Anderssen RS (eds) MODSIM2011, 19th international congress on modelling and simulation. Modelling and Simulation Society of Australia and New Zealand, December 2011, pp 352–358, http://www.mssanz.org.au/modsim2011/A3/shi.pdf
Sinclair ER, Alder J (1985) Development of a computer simulation model of stored product insect populations on grain farms. Agric Syst 18:95–113
Stansfield WD (1991) Schaum's outline of theory and problems of genetics, 3rd edn. McGraw-Hill, New York
Tabashnik BE, Croft BA (1982) Managing pesticide resistance in crop-arthropod complexes: interactions between biological and operational factors. Environ Entomol 11:1137–1144
Acknowledgements
The authors would like to acknowledge the support of the Australian Government’s Cooperative Research Centres Program. We also thank Rob Emery and Yonglin Ren and the GRDC for their great help in provision of raw data and information about beetle life cycles and silo fumigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Athanassiou.
Appendices
Appendix 1
Figures for the original two-locus model
Table for resistance factors
See Table 8.
Appendix 2
Log-normal distribution as a model of time within a life stage
Log-normal distribution is a continuous distribution in which the logarithm of a variable has a normal distribution (Limpert et al. 2001). It is appropriate for modelling the time an individual spends within a given life stage because it is bounded below at zero, and because it can be parameterized to have a non-zero median and to simultaneously give a restricted range of likely values that does not necessarily include values close to zero (unlike the Weibull distribution for example).
The probability density function (pdf) and cumulative distribution function (cdf) for the log-normal distribution are, respectively,
where μ and σ are the mean and std of the corresponding normal distribution, respectively, and erf(x) is the complementary error function.
Given the mean and std values of a sample L from a random variable T with a log-normal distribution, M L and S L, the parameters μ and σ can be estimated as follows. The expectation E(T) and variation Var(T) are the estimates of M L and (S L)2, respectively, and we know E(T) and Var(T) are
Substituting (16) into (17) we have
Thus, given the expected mean and standard deviation of the times spent within different life stages by different individuals, we have a simple method to calculate the parameters of the log-normal distribution used to stochastically generate the actual time spent within a given life stage by a given individual.
For model verification, we used the Python built-in function lognormvariate(μ,σ) to generate 10,000 random numbers from log-normal distribution with mean M L and std S L for the life duration of each stage of the beetle. The results are listed in Table 9.
Rights and permissions
About this article
Cite this article
Shi, M., Renton, M., Ridsdill-Smith, J. et al. Constructing a new individual-based model of phosphine resistance in lesser grain borer (Rhyzopertha dominica): do we need to include two loci rather than one?. J Pest Sci 85, 451–468 (2012). https://doi.org/10.1007/s10340-012-0421-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-012-0421-6