Abstract
The preparation of linear polyacrylamide (LPA)-coated capillary is a multistep, laborious process. In this study, five different LPA-coating procedures were compared regarding the capillary pretreatment (incubation time and temperature), the formation of the intermediate silyl layer and the attachment of the polymer top layer. LPA coatings were examined by the analysis of a standard mixture solution of insulin, rituximab and hemoglobin using a background electrolyte (BGE) of 50 mM HCOOH (pH 2.6). The results were contrasted with those that were obtained using the bare fused-silica capillary. The reproducibility of the simplest LPA-coating preparation was tested. The long-term stability of the capillary coating was evaluated through inter-day precision data using a large number of injections of the three-protein test mixture solution. The implementation of a short-term procedure at elevated temperature, guided by a comprehensive review of various conditions documented in the literature, yielded an efficiently functional LPA coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
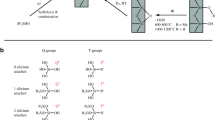
Among the other capillary electrophoresis (CE) techniques, capillary zone electrophoresis (CZE) is frequently applied for protein analysis due to its many appealing features, including high separation efficiency and low sample consumption [1]. During CZE, the application of dynamic and permanent coatings for the modification of the bare fused-silica inner surface is often required to avoid protein-wall interactions and improve selectivity as well as reproducibility [2, 3]. The permanent coating agents are not incorporated in the BGE, and they can be classified as physical and covalent types based on the attachment [4, 5]. The permanent covalent coatings irreversibly attach to the capillary wall and ensure long-term stability, overcoming the need for surface regeneration and giving the possibility for MS detection [2, 3, 6]. LPA is one of the most preferred hydrophilic coating and is usually optimal for the separation of proteins [6,7,8,9]. LPA was used for the first time by Hjertén in 1985 [10]. Since its introduction, Hjertén’s coating has undergone a number of modifications and enhancements. Different protocols can be found in the literature regarding the three main steps (etching, silanization and polymerization) in Table 1. [2, 3, 6]. The results of coating are strongly affected by the capillary pretreatment; the surface must be activated by etching and leaching with sodium hydroxide (NaOH) and hydrochloric acid (HCl), respectively. The dehydration of capillary improves the yield of silylation by removing water from the surface, which hinders the formation of the intermediate layer [11, 12]. Cifuentes et al. performed an overnight dehydration process using high temperature (160 °C) [13, 14], while Courtois et al. improved the etching effect by 3 h of incubation with 1 M NaOH at 120 °C where dehydration was conducted at 120 °C in an oven for 1 h [12]. Grignard’s reagent as an anchor was used for surface derivatization as an alternative to siloxane bonds. The formation of the Si–C bond exhibited greater hydrolytic stability, allowing the application of extreme pH values. However, its drawback was the production of precipitate that blocked the capillary lumen [11, 15].
Since water hinders silanization, the presence of water in the silanization step was shown to be a major factor contributing to capillaries of poor quality. Curtois et al. tried 11 different types of silanization protocols [12]. The best silanization was based on silyl reagent in organic solvent either at room or elevated (120 °C) temperature [12]. A longer incubation time for silanization (12–24 h) was applied by several researchers [13, 14, 16, 17], where the intermediate layer of the capillary coating was functionalized with C=C bonds. In this scenario, a longer incubation resulted in a better capillary coating due to a more complete reaction. On the other hand, a short-time silanization procedure was also reported by Guttman et al. [18].
A mixture solution of acrylamide (AA) (1–4% (w/v)), tetramethylethylenediamine (TEMED) (0.0025–0.1% (v/v)), and ammonium persulfate (APS) (0.0025–0.1% (w/v)) in varying concentrations was used to form the polymer top layer (Table 1). The effects of concentration changes were elaborated by Cifuentes. The higher the monomer concentration and the lower the initiator concentration are, the larger the polymer size [14]. In Dovichi and McCool’s application, no TETA was employed for polymerization [16, 17]. An automated LPA-coating procedure was developed where the APS reagent was introduced by voltage into the capillary filled with the monomer solution of acrylamide [18].
In this work, we compared five coating procedures performed in the CE instrument, each generated with a different incubation time and temperature during capillary pretreatment, silanization and polymer top layer formation procedures. LPA preparations were the subject of many investigations, but this type of comparison has not yet been performed. The aim of this research was to demonstrate the efficacy of the LPA coating, which was found to be the most straightforward and efficient for the separation of a protein mixture of rituximab, insulin and hemoglobin. These test proteins were selected because they exhibit considerable differences from one another (in terms of isoelectric point (pI) and molecular weight). Moreover, rituximab and hemoglobin possess multiple isoforms that are challenging to separate with CZE. In the literature, there are numerous contrasting approaches and conditions, however, our investigations focused on simplifying the preparation of LPA-coated capillary and providing a simple alternative to its commercially available counterparts by avoiding the remarkably long incubation times and high temperature.
Materials and Methods
Chemicals
All chemicals were of analytical grade. HCl, NaOH, 3-(trimethoxysilyl)propyl methacrylate, or its alternative names γ-methacryloxypropyltrimethoxysilane and 3-trimethoxysilylpropyl 2-methylprop-2-enoate (γ-MAPS, CAS number: 2530-85-0), acetone, AA, TEMED, APS, tris(hydroxymethyl)aminomethane hydrochloride (TRIS–HCl), formic acid (HCOOH), acetic acid (CH3COOH), ammonium acetate, dimethyl sulfoxide (DMSO) and hemoglobin were obtained from Sigma Aldrich (St. Louis, MO). The dilutions were prepared prior to use in MilliQ water (Millipore Synergy UV). Human insulin (Humulin R, 3.5 mg/mL) was purchased from Lilly (France), rituximab (MabThera, 10 mg/mL) was obtained from Hoffmann-LaRoche (Switzerland). For the preparation of other BGEs, HCOOH was diluted to 50 mM, solid ammonium acetate was dissolved and its pH was adjusted to the desired value by titration with glacial acetic acid and a 32% ammonium hydroxide solution. To adjust the pH (pH 7) of TRIS–HCl (0.1 M), concentrated HCl was added following its dissolution.
Instrumentation
CE separations were carried out using 7100 CE System (Agilent, Waldbronn, Germany) coupled to a UV detector. Separations were performed using a bare fused-silica capillary (Polymicro, Phoenix, USA, TSP050375 3, 363–10 (Part 1068150017)) of 65 cm × 50 µm i.d., 360 μm o.d., and an LPA-coated capillary of 65 cm × 50 µm i.d., 75 cm × 50 µm i.d. and 85 cm × 50 µm i.d., 360 μm o.d.. The precondition procedures for bare fused-silica capillary were 1 M NaOH for 5 min, 1 M HCl for 5 min and BGE for 8 min, while for LPA-coated capillary, it was only BGE for 5 min. Hydrodynamic sample injection (50 mbar × 2 s) was carried out at the anodic end of the fused-silica and LPA-coated capillaries. For the electrophoretic separation, 30 kV was used and UV detection was performed on-capillary at a wavelength of 200 nm. Chemstation version B.04.02 software (Agilent) was used for operating the CE instrument and for processing the results.
Preparation of LPA Coatings
The preparation of the LPA static coating was based on the instructions by Dovichi [16] and McCooll [17], with a few small modifications (Table 2). Every solution was freshly prepared prior to the coating procedure. Silyl reagent was a 1:1 (v/v) mixture of γ-MAPS and acetone. For the top layer, the solutions were prepared as follows: 3 ml acrylamide (AA) of 4% (w/v) in 0.1 M TRIS–HCl (pH 7) buffer solution was prepared and put under vacuum for 5 min to remove oxygen; APS was dissolved in water to the final concentration of 10% (w/v). The coating reagents (LPA solution) were prepared in CE vials (2 × 1 mL): 1 µL TEMED and 10 µL 10% (w/v) APS were added into 1 mL 4% (w/v) degassed AA solution. This LPA solution was prepared right before the flushing of the capillary.
Results and Discussion
CZE Separations in Coated and Uncoated Capillaries
The separation of intact proteins in bare fused-silica capillary requires the application of electrolytes with strong acidic or alkalic pH values. At very high pH, the silanol groups on the capillary wall are deprotonated, resulting in a negatively charged surface. Similarly, the proteins possess a net negative charge when their pI is below the empirical pH. Conversely, at very low pH, both the silanol groups and the proteins are protonated, leading to a neutral silica surface and positively charged proteins (when their pI is above the pH). In both cases, the electrical repulsion reduces the adsorption of the protein on the capillary wall [19,20,21].
Bare fused-silica (Fig. 1a, b) and LPA-coated (Fig. 1c, the LPA coating was prepared using the procedure outlined in the Case II (extended procedure at elevated temperature)) capillaries have been compared for the separation of rituximab with the BGE at pH 2.6, which was suitable for both capillaries. In bare fused silica, narrow peak shape of rituximab was expected, however, the peak broadening caused by a significant wall adsorption (Fig. 1a) could not be improved even by tenfold dilution of the rituximab solution (Fig. 1b). Analysis of diluted protein yielded smaller yet tailed peak shape. Nevertheless, the application of even lower pH values may have resulted in the denaturation and precipitation of the protein. In case of LPA-coated capillary, extreme pH values (pH < 2, pH > 8) are also not suitable due to the hydrolytic instability of the coating [22].
The CZE electropherograms of rituximab using bare fused-silica (a, b) and LPA-coated (c) capillaries. Conditions: capillaries ltot: 65 cm × 50 µm i.d., leff: 57 cm, BGE: 50 mM HCOOH (pH 2.6), separation voltage: 30 kV, injection: 50 mbar × 2 s, preconditioning: 5 min 1 M NaOH, 5 min 1 M HCl, 8 min BGE washing (a, b), 5 min BGE washing (c), detection wavelength: 200 nm. Sample: rituximab 10 mg/mL (a), 1 mg/mL (b, c)
The adsorption of rituximab to the capillary surface was considerably decreased in LPA-coated capillary, consequently resulting in improved separation efficiency. Rituximab was resolved into one basic and two–three acidic variants (Fig. 1c). The acidic variants could be formed by glycosylation with sialic acid or by deamidation of asparagine or glutamine, whereas the basic variants could be due to the formation of Fc-1 lysine (1-K) or Fc-2 lysine (2-K) (Fig. 2c). The peak patterns of basic/acidic variants were consistent with our previous results using dynamic coating with a running buffer containing 800 mM EACA-2 mM TETA-0.05% HPMC (pH 5.2) [23]. Analysis of the diluted rituximab (1 mg/mL) in LPA-coated capillary (Fig. 1c) yielded the same peak height that was obtained analyzing the concentrated sample (10 mg/mL) in bare fused-silica capillary (Fig. 1a). This phenomenon was initiated by the strong wall interaction and peak broadening in uncoated capillary. Although comparable migration times were anticipated in both capillaries, rituximab migrated faster in the coated capillary compared to the uncoated one (Fig. 1a, c). This might be explained by the gradual absorption of the positively charged protein on the bare fused-silica capillary wall, generating a continually rising, counter directed EOF.
The comparison of bare fused-silica (a) and LPA-coated (b, c) capillaries for the separation of the mixture of rituximab, hemoglobin and insulin. c is same as b but it is presented in a narrower scale. The analysis conditions for a and b, c were the same as stated at Fig. 1a and Fig. 1c, respectively. Sample: 1: rituximab (1 mg/mL), 2: hemoglobin (3 mg/mL), 3: insulin (1.16 mg/mL)
A similar result was observed when a mixture of three proteins was analyzed. The remaining two proteins, insulin and hemoglobin, significantly differ from rituximab regarding molecular weight and pI value. For these proteins, the CZE signal of the bare fused-silica capillary exhibited considerable broadening and peak tailing (Fig. 2a). Moreover, two variants of hemoglobin were merged with the first proteoform (Fig. 2a). The separation power for different protein variants (hemoglobin subunits and rituximab charge variants) has been improved by LPA coating (Fig. 2b, c) due to its selectivity-enhancing property. Nevertheless, LPA-coated capillary appeared to have very little effect on the triangular peak shape of insulin. This was reminiscent of the electrodispersion phenomenon that is characterized by a triangular peak shape due to the notable difference between the mobilities of the protein and the electrolyte ion (Fig. 2a–c).
Comparison of Different LPA-Coating Processes
The procedures for the preparation of LPA-coated capillaries are elaborated in “Preparation of LPA Coatings” (Table 2). The efficacy of a coating procedure was verified by analyzing a DMSO solution (a neutral component). Since the capillary surface is covered by the neutral polyacrylamide, during the extended electrophoresis run at pH 7, no peak of DMSO appeared in case of successful coating, in contrast to bare fused-silica capillary where the sharp DMSO peak was obtained (Fig. S1). It is of significance to note that, in the case of successful execution of the coating process, the electrophoretic mobility of EOF was negligible (lower than 4.1 × 10–5 cm2/Vs), while the residual EOF was present. This residual EOF can be effectively assessed using Williams’ methodology, as outlined in [24]. The application of procedure (I) (long-time etching, silanization and polymerization, performed at room temperature) produced an incomplete coating. The separation of hemoglobin variants failed (Fig. S2a) and considerable EOF was generated, as evidenced by the appearance of the DMSO peak during the extended analysis time (Fig. S2b), indicating the insufficient coverage of the capillary wall and the presence of free silanol clusters. The magnitude of EOF was lower in this LPA-coated capillary compared to that of fused silica (Fig. S2c).
The coating procedures I and II differed solely in the temperature applied during the three main steps of preparation. In procedure II, the cassette temperature was increased to 60 °C and maintained at that level throughout the entire incubation period. The cassette remained in the instrument during the coating process. By utilizing LPA capillary prepared at this elevated temperature, a noticeable improvement in separation efficiency was achieved. This was demonstrated by the successful resolution of different hemoglobin subunits and charge variants of rituximab (Fig. 3a). The elevated temperature resulted in better coating formation, and therefore, its continued implementation in the subsequent four experiments.
The comparison of LPA-coated capillaries prepared in different ways (a: II-, b: III-, c: IV- d: V-coating procedure). The analysis conditions were the same as stated at Fig. 1c. Sample (a–d): 1: rituximab (1 mg/mL), 2: hemoglobin (3 mg/mL), 3: insulin (1.16 mg/mL)
During the procedures III–V, the flushing and incubation time of successive steps were reduced. These steps included etching in procedure III, etching + silanization in procedure IV and etching + silanization + polymerization in procedure V. Analyzing the mixture solution of three proteins, different coating procedures achieved an identical peak pattern. The separation efficiency of four LPA-coated capillaries remained consistent, with the rituximab’s main peak resolving into 4 variants and hemoglobin’s main peak resolving into two variants. In addition, insulin maintained its characteristic triangular shape (Fig. 3a–d). Notably, performing the short-time coating at 60 °C demonstrated the comparable effectiveness to the long-time coating procedure at the same temperature. However, the long-coating procedure conducted at room temperature did not yield a satisfactory LPA-coating quality.
Analytical Performance
The repeatability of the short-time coating (V. type) preparation was investigated. Six capillaries were prepared on different days and their separation efficiencies were tested by analyzing a solution containing hemoglobin and rituximab (Fig. 4). Only these two molecules were used to assess the efficiency of the LPA-coated capillaries, since the functional coating should exhibit a characteristic peak pattern. The resolution of isoforms serves as an indicator of the LPA-coating quality, as the main peaks of rituximab and hemoglobin were not resolved from variants in an uncoated bare fused-silica capillary (Fig. 2a). The results showed that all six LPA-coated capillaries produced the same separation efficiency (Fig. 4), indicating good repeatability in coating preparation with the shortened procedure. A reduction in the preparation time from 27 to 4 h did not compromise the efficiency of the LPA layer.
A repeatability evaluation of the LPA-coating preparation (a–f), prepared by short procedure at 60 °C. The analysis conditions were the same as stated at Fig. 1c. Sample: 1: rituximab (1 mg/mL), 2: hemoglobin (3 mg/mL)
In terms of precision, good intra- and inter-day repeatability data (0.11–0.35 RSD% and 1.72–4.75 RSD% for migration times and peak areas, respectively) were obtained in LPA-coated capillary (V. type), even when using only BGE for preconditioning (Table 3). LPA-coated capillary demonstrated greater stability in separations compared to bare fused-silica capillary (Fig. S3). While significant wall adsorption occurred after 15. injection in bare fused-silica capillary (Fig. S3c, d), the LPA coating maintained its surface quality (Fig. S3a, b).
The long-term stability of the LPA coating was also evaluated. The coating was found to be highly stable for at least 100 runs (Fig. S4). No wall adsorption was observed and the migration time shifts were attributed to changes in BGE properties (pH, ionic strength) caused by electrolysis at the electrodes during electrophoresis. Refreshing the BGE following every 20th run helped mitigate these effects. Improved precision could be achieved using the replenishment system of the CE instrument. The shortened coating procedure (V. type) was also evaluated using longer capillaries (75 cm and 85 cm) to assess its applicability in coupling CE with MS. Remarkably, the same flushing and reagent application protocol proved to be sufficient even for longer capillaries. Increasing the length of the LPA-coated capillary resulted in the appearance of an additional isoform of rituximab and improved resolution between hemoglobin subunits (Fig. S5). This finding highlights the effectiveness of the short-time LPA-coating procedure in longer capillaries, which holds significant importance for CE–MS applications that necessitate the use of extended capillary lengths.
Conclusion
This study examined five different types of LPA coatings, focusing on variations in reagent flushing time, incubation duration and temperature in an effort to streamline the complex, laborious coating procedure. The use of elevated temperature proved crucial for successful LPA preparation, as the coating was ineffective at room temperature. Notably, reducing the coating time did not compromise the efficiency of the coating, as evidenced by the generation of identical electropherograms across the four different LPA capillaries. The proposed coating procedure, involving shortened etching, silanization, polymerization steps performed at 60 ℃ demonstrated reliable performance and yielded well-functioning coatings. The significant reduction in the conventional long-time procedure from 27 to 4 h represents a notable time saving. The efficiency and stability of the LPA coating were evaluated using a three-protein test mixture of rituximab, hemoglobin and insulin, with a BGE composed of 50 mM HCOOH (pH 2.6). The standard deviations of the migration times and peak areas were less than 1 and 5 RSD%, respectively. The LPA coating demonstrated remarkable separation power, particularly in the effective separation of different isoforms of proteins (charge variants of rituximab, subunits of hemoglobin). Moreover, the resulting capillary coating exhibited excellent stability, with consistent performance observed at least for a hundred consecutive runs.
Data Availability
Data are available from the authors upon request.
Abbreviations
- LPA:
-
Linear polyacrylamide
- BGE:
-
Background electrolyte
- CE:
-
Capillary electrophoresis
- CZE:
-
Capillary zone electrophoresis
- NaOH:
-
Sodium hydroxide
- HCl:
-
Hydrochloric acid
- DMSO:
-
Dimethyl sulfoxide
- AA:
-
Acrylamide
- TEMED:
-
Tetramethylethylenediamine
- APS:
-
Ammonium persulfate
- pI:
-
Isoelectric point
- TRIS–HCl:
-
Tris(hydroxymethyl)aminomethane hydrochloride
- HCOOH:
-
Formic acid
- γ-MAPS:
-
3-Trimethoxysilyl)propyl methacrylate, γ-methacryloxypropyltrimethoxysilane, 3-trimethoxysilylpropyl 2-methylprop-2-enoate
- THF:
-
Tetrahydrofuran
- DMF:
-
Dimethylformamide
- N:
-
Number of theoretical plates
References
Dawod M, Arvin NE, Kennedy RT (2017) Recent advances in protein analysis by capillary and microchip electrophoresis. Analyst. https://doi.org/10.1039/c7an00198c
Rodriguez I, Li SFY (1999) Surface deactivation in protein and peptide analysis by capillary electrophoresis. Anal Chim Acta 383:1–26. https://doi.org/10.1016/S0003-2670(98)00485-1
Hajba L, Guttman A (2017) Recent advances in column coatings for capillary electrophoresis of proteins. Trends Anal Chem 90:38–44. https://doi.org/10.1016/j.trac.2017.02.013
Katayama H, Ishihama Y, Asakawa N (1998) Stable capillary coating with successive multiple ionic polymer layers. Anal Chem 70:2254–2260. https://doi.org/10.1021/ac9708755
Weinbauer M, Stutz H (2010) Successive multiple ionic polymer layer coated capillaries in the separation of proteins—recombinant allergen variants as a case study. Electrophoresis 31:1805–1812. https://doi.org/10.1002/elps.201000077
Horvath J, Dolnik V (2001) Polimer wall coatings for capillary electrophoresis. Electrophoresis 22:644–655. https://doi.org/10.1002/1522-2683(200102)22:4%3c644::AID-ELPS644%3e3.0.CO;2-3
Hunta G, Hotalingb T, Chena AB (1998) Validation of a capillary isoelectric focusing method for the recombinant monoclonal antibody C2B8. J Chromatogr A 800:355–367. https://doi.org/10.1016/S0021-9673(97)01134-5
Graf M, Garcia RG, Wätzig H (2005) Protein adsorption in fused-silica and polyacrylamide-coated capillaries. Electrophoresis 26:2409–2417. https://doi.org/10.1002/elps.200410360
Suratman A, Wätzig H (2007) Reproducible protein analysis by CE using linear polyacrylamide-coated capillaries and hydrochloric acid rinsing. Electrophoresis 28:2324–2328. https://doi.org/10.1002/elps.200700108
Hjerten S (1985) High-performance electrophoresis, elimination of electroendosmosis and solute adsorption. J Chromatography 347:191–198. https://doi.org/10.1016/S0021-9673(01)96347-2
Cobb KA, Dolnik V, Novotny M (1990) Electrophoretic separations of proteins in capillaries with hydrolytically stable surface structures. Anal Chem 62:2478–2483. https://doi.org/10.1021/ac00221a013
Courtois J, Szumski M, Bystr E, Iwasiewicz A, Shchukarev A, Irgum K (2006) A study of surface modification and anchoring techniques used in the preparation of monolithic microcolumns in fused silica capillaries. J Sep Sci 29:14–24. https://doi.org/10.1002/jssc.200500294
Cifuentes A, Canalejas P, Ortega A, Diez-Masa J-C (1998) Treatments of fused-silica capillaries and their influence on the electrophoretic characteristics of these columns before and after coating. J Chromatogr A 823:561–571. https://doi.org/10.1016/S0021-9673(98)00295-7
Cifuentes A, Canalejas P, Diez-Masa J-C (1999) Preparation of linear polyacrylamide-coated capillaries, study of the polymerization process and its effect on capillary electrophoresis performance. J Chromatogr A 830:423–438. https://doi.org/10.1016/S0021-9673(98)00923-6
Gelfi C, Curcio M, Righetti PR, Sebastiano R, Citterio A, Ahmadzadeh H, Dovichi NJ (1998) Surface modification based on Si-0 and Si-C sublayers and a series of N-substituted acrylamide top-layers for capillary electrophoresis. Electrophoresis 19:1677–1682. https://doi.org/10.1002/elps.1150191026
Zhu G, Sun L, Dovichi NJ (2016) Thermally-initiated free radical polymerization for reproducible production of stable linear polyacrylamide coated capillaries, and their application to proteomic analysis using capillary zone electrophoresis–mass spectrometry. Talanta 146:839–843. https://doi.org/10.1016/j.talanta.2015.06.003
McCool EN, Lubeckyj R, Shen X, Kou Q, Liu X, Sun L (2018) Large-scale top-down proteomics using capillary zone electrophoresis tandem mass spectrometry. J Vis Exp 140:5–10. https://doi.org/10.3791/58644
Bodnar J, Hajba L, Guttman A (2016) A fully automated linear polyacrylamide coating and regeneration method for capillary electrophoresis of proteins. Electrophoresis 37:3154–3159. https://doi.org/10.1002/elps.201600405
Stutz H (2009) Protein attachment onto silica surfaces—a survey of molecular fundamentals, resulting effects and novel preventive strategies in CE. Electrophoresis 30:2032–2061. https://doi.org/10.1002/elps.200900015
Hamidli N, Andrasi M, Nagy C, Gaspar A (2021) Analysis of intact proteins with capillary zone electrophoresis coupled to mass spectromery using uncoated and coated capillaries. J Chromatogr A 1654:462448. https://doi.org/10.1016/j.chroma.2021.462448
Tran NT, Myriam Taverna M (2016) Capillary electrophoresis of proteins and peptides methods and protocols. Humana, New York. https://doi.org/10.1007/978-1-4939-4014-1
Beneito-Cambra M, Anres P, Vial J, Gareil P, Delaunay N (2016) Stability and effectiveness of linear polyacrylamide capillary coating to suppress EOF in acidic media in the presence of surfactants, ionic liquids and organic modifiers. Talanta 150:546–552. https://doi.org/10.1016/j.talanta.2015.12.070
Andrasi M, Gyemant G, Gaspar A (2014) Analysis of rituximab, a therapeutic monoclonal antibody by capillary zone electrophoresis. J Chromatogr Sep Tech 6:1–8. https://doi.org/10.4172/2157-7064.1000259
Williams BA, Gy V (1996) Fast, accurate mobility determination method for capillary electrophoresis. Anal Chem 68:1174–1180. https://doi.org/10.1021/ac950968r
Acknowledgements
The authors acknowledge the financial support provided to this project by the National Research, Development and Innovation Office (K142134), Hungary.
Funding
Open access funding provided by University of Debrecen. The research presented in the article was carried out within the framework of the National Research, Development and Innovation Office (K142134), Hungary.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MA and BZ. The first draft of the manuscript was written by MA, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors of this paper does has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andrasi, M., Zagyi, B. & Hamidli, N. Study of Effect of Incubation Time and Temperature on the Linear Polyacrylamide-Coating Performance for the Separation of Proteins by Capillary Zone Electrophoresis. Chromatographia 86, 659–668 (2023). https://doi.org/10.1007/s10337-023-04276-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04276-x