Abstract
UPLC-MS/MS method was developed for determination of bioactive compounds including chlorogenic acid (CA), apigenin (AP) and apigenin 7-glucoside (AP7G) in goat serum. The analytes were separated on Kinetex C18 analytical column and a mobile phase consisting 0.1% formic acid in water and methanol with gradient elution. Detection of analytes was performed using a triple quadrupole mass spectrometer with electrospray ionization in positive mode. The lower limit of quantitation was 0.5 ng mL–1 for AP and AP7G and 2.5 ng mL–1 for CA. The inter- and intra-day precision of the method expressed as relative standard deviation was in the range of 1.22–14.5%. The accuracy of the method expressed as the relative error was in the range of 0.147–14.6%. The extraction recoveries of the compounds were 76.2–89.5%. The validated method was successfully applied for analysis of the compounds in the serum of sixty goats, half of which were fed with fodder with the addition of an herbal mixture. The higher concentration values of all analyzed compounds were reported in the serum of the animals fed with herbs compared to the control group receiving standard fodder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several plant bioactive polyphenols may play a remarkable role in preventing many chronic diseases. The largest group of naturally occurring polyphenols are flavonoids, including apigenin (AP) (Fig. 1a). The compounds possess antioxidant, anti-inflammatory, anticancer properties, and are able to inhibit platelet aggregation, reduce plasma levels of low-density lipoproteins and cell proliferation [1]. AP is present principally as glucosides in herbs (chamomile, thyme), vegetables, fruits, and plant-based beverages [2]. After ingestion but before absorption, AP glucosides, including apigenin 7-glucoside (AP7G) (Fig. 1b), are metabolized to the aglycone by β-glucosidases in the stomach and small intestines or within endogenous colon microflora [3]. A study in a rat model suggests variability in the rate of deglycosylation in the gastrointestinal tract, which may affect the tissue distribution of AP and its bioactivity [4].
Chlorogenic acid (CA) (Fig. 1c) is a polyphenolic compound produced by plants through the shikimic acid pathway during aerobic respiration. It is present at high concentrations in green coffee, green tea, fruits, vegetables and honeysuckle. Its biological activities include anti-oxidant, anti-inflammatory, anti-bacterial, and anti-tumor actions, liver and kidney protection, regulation of glucose and lipid metabolism, protection of the nervous system, and vasodilatory effect on blood vessels [5]. CA is immediately absorbed in stomach and upper gastrointestinal tract, and may be slowly absorbed throughout the small intestine. The absorbed compound is metabolized via conjugation, hydrogenation, and \(\alpha\)– or \(\beta\)-oxidation. It can be also subjected to gut microbiota-mediated metabolism [6]. The study on rats revealed that the bioavailability of CA is closely related to the metabolic capacity of the organism’s gut flora [7]. In view of the biological activity of AP and CA, it is of great importance to improve the content of the compounds in plants and to analyze their bioavailability in animals and humans.
The abovementioned polyphenols, including AP, AP7G, and CA, were chosen based on our previous study [8], and they served as the indicators for assessing the effect of an herbal feed additive on the health of lactating goats. We observed the positive effect of a herbal supplement on the composition of the goat gut microbiota expressed by lactic bacteria count [8]. Up to now, there is no study on the AP, AP7G and CA concentrations in goats. Nevertheless, several reports are available regarding the analysis of the polyphenolic compounds in other animal species, including rats [9,10,11,12,13,14,15], and mice [16, 17]. In these reports, validated LC–MS/MS methods were applied due to the high sensitivity and specificity of the technique.
The aim of the presented research was to develop a sensitive and reliable UPLC-MS/MS method for the determination of AP, AP7G, and CA in the blood serum of goats. The method was applied to analyze the chosen bioactive compounds in 60 lactating domestic goats (Capra hircus), which provide the raw material for the commercial production of dairy products. The animals were fed with standard fodder and an additional supplement containing nine herbs to the received feed, just like in our previous work, leading to improved animal health [8].
Materials and Methods
Chemicals and Reagents
Chlorogenic acid (CA, purity ≥ 95%), apigenin 7-glucoside (AP7G, purity ≥ 97%) and formic acid (FA, purity ≥ 95%) were obtained from Merck (Darmstadt, Germany). Apigenin (AP, purity ≥ 98%) was from Pol-Aura (Dywity, Poland), and apigenin-d5 (APd5, ≥ 98%) was from Santa Cruz Biotechnology (Dallas, Texas, USA). Acetonitrile, methanol, and water of LC/MS grade were purchased from Witko (Lodz, Poland).
UPLC–MS/MS Chromatographic Conditions
The study was performed using a Shimadzu UPLC Nexera set (Shimadzu Co., Kyoto, Japan) equipped with five-channels degasser (DGU-20A5) and thermostated autosampler (SIL-30AC). UPLC was coupled with a triple quadrupole mass spectrometer (LCMS-8030). The Labsolution Series Workstation system (Shimadzu, Kyoto, Japan) has been used to process the data.
The separation of the analytes (AP, AP7G, CA) and APd5 used as an internal standard (IS) was performed in Kinetex® C18 analytical column (100 mm × 2.1 mm, 2.6 μm) attached to security guard ultra-cartridge (Phenomenex, Torrance, CA, USA). The column temperature was kept at 25 °C by CTO-2AC column oven (Shimadzu, Kyoto, Japan). The mobile phase consisted of water (A) and methanol (B) both containing 0.1% (v/v) FA with flow rate adjustment (0.25 mL min–1) at gradient elution: 0–7 min 50–90% phase B, 7–9 min 90–50% phase B, 9–11 min 50% phase B. The injected sample volume was 5 μL. Positive electrospray ionization mode (ESI+) was used for eluent introduction from the UPLC column to the MS interface. The electrospray needle voltage was maintained at 4.5 kV. MS interface was adjusted with the following parameters: desolvation line 250 °C, heat block temperature 400 °C, interface temperature 350 °C, nitrogen was used as the drying gas and as the nebulizing gas with flow rates of 12 and 2 L/min respectively. The parameters of LC–MS/MS analysis are presented in Table 1.
For carry-over effect minimization, a mixture of methanol–water (50:50, v/v) was used to rinse the autosampler needle before and after sample aspiration. Ion transitions of the tested compounds were observed in the MRM mode, and their identification was based on the ratio of the mass of the obtained ions to their charges (m/z). For the quantitative analysis, the most intense precursor-to-product transitions were selected: 270.7 → 152.9 for AP, 432.8 → 271.0 for AP7G, 275.8 → 122.1 for APd5, and 354.8 → 162.9 for CA (Table 2).
Standard Solutions
Stock solutions of the three analytes, AP, AP7G and CA at a concentration of 10 μg mL–1 and stock solution of APd5 at a concentration of 10 μg mL–1 were prepared in methanol. Standard solutions of the analytes were prepared from the stock solutions in methanol at concentrations: 2; 5; 10; 25; 50; 100; 250; and 500 ng mL–1 of AP and AP7G, 10; 25; 50; 100; 250; 500; 750 and 1000 ng mL–1 of CA and 1000 ng mL–1 of APd5. All solutions were stored at − 20 °C until analysis.
Sample Preparation for Method Validation
For the method validation, a goat serum with undetectable AP, AP7G and CA was selected. An aliquot of 200 μL of blank serum was transferred to a glass vial followed by the addition of 20 μL of the analytes standard solution and 20 μL of the IS solution. The obtained concentrations of the analytes in goat serum were as follows: 0.2; 0.5; 1; 2.5; 5; 10; 25; and 50 ng mL–1 of AP and AP7G, 1; 2.5; 5; 10; 25; 50; 75 and 100 ng mL–1 of CA and 100 ng mL–1 of APd5. Then, aliquots of 800 μL of acetonitrile containing 0.1% formic acid for precipitation were added and the samples were vortexed for 3 min. Subsequently, the samples were cooled for 10 min at − 20 °C and centrifuged for 10 min at 14000 g. 900 μL of supernatant was transferred to glass vials and evaporated at 45 °C under vacuum (Concentrator plus, Eppendorf, Enfield, USA). A 200 μL of water–methanol (50:50, v/v) solution containing 0.1% FA was used to dissolve the dry residue. An aliquot (5 μL) was injected directly into the UPLC–MS/MS system.
Samples obtained from goats were prepared according to the same procedure with the exception that 20 μL of methanol instead of the standard solution was added to 200 μL of the animal serum.
Method Validation
The method's selectivity was evaluated by analyzing the chromatograms of blank serum samples with undetectable AP, AP7G, and CA, taken from six individual goats not fed with herbal supplements. The samples were compared with those containing IS and analytes at the LLOQ level to check the potential interferences at the retention times.
Carry-over was evaluated by injecting the blank sample immediately after injection of the calibrator at the highest concentration of the analytes.
Linearity of the calibration curves of AP, AP7G and CA were estimated for the ratio of the analyte peak areas to that of the APd5, as a function of the analyte concentration in serum samples. Mandel’s fitting test has been applied for the evaluation of the linearity of a straight line regression model. Equations of the calibration curves were used for calculating the concentrations of analytes in goat serum samples.
The determination of Limit of Detection (LOD) and Lower Limit of Quantification (LLOQ) was based on standards of known concentration of each analyte and observing the peak signal with the corresponding signal to noise ratio (S/N). LLOQ was defined as the lowest concentration of the analytes determined by the method within the relative error (%RE) and relative standard deviation (%RSD) not exceeding 20%. LOD was defined as the smallest concentration of analyte that can be detected with S/N greater than 3:1.
Quality control samples (QCs) were prepared at concentrations: LLOQ (0.5 ng mL–1 of AP and AP7G, and 2.5 ng mL–1 of CA), low (1 ng mL–1 of AP and AP7G, and 5 ng mL–1 of CA), medium (10 ng mL–1 of AP, AP7G, and CA) and high (50 ng mL–1 of AP, AP7G, and 100 ng mL-1 CA). To determine the inter-day and intra-day precision and accuracy of the method, QCs were analyzed over five consecutive days and in five replicates on the same day, respectively. The precision was calculated as %RSD from the formula:
Accuracy was expressed as the percent difference between the mean determined concentration and the nominal concentration. Recovery of three analytes from the serum was evaluated by analyzing five replicates of low (1 ng mL–1 of AP and AP7G, and 5 ng mL–1 of CA), medium (10 ng mL–1 of AP, AP7G, and CA) and high (50 ng mL–1 of AP, AP7G and 100 ng mL–1 of CA) concentrations for each analyte. Two sets of serum (Series I and Series II) have been used to determine the recovery of the method. Series I consisted of spiked serum samples with the analytes and the IS whereas, series II contained spiked serum samples with the pure methanol and the APd5. The extraction of Series I/II have been performed in accordance with sample preparation for method validation described in Sect. “Sample preparation for method validation”. Dry residues of samples from series I have been dissolved in 200 μL of water–methanol (50:50, v/v) solution containing 0.1% FA. Dry residues of samples from series II were dissolved in 200 μL of the analytes solutions at the same concentrations as those of Series I prepared in the water–methanol (50:50, v/v) mixture containing 0.1% FA. Recovery was calculated as (CI-series)/(CII-series)·100%.
Matrix effect was studied using serum samples spiked with low (1 ng mL–1 of AP and AP7G, and 5 ng mL–1 of CA) and high (50 ng mL of AP, AP7G and 100 ng mL–1 of CA) concentrations of the analytes. It was expressed by matrix factor (MF) calculated by dividing the peak measured by analyzing blank matrix spiked with analytes after protein precipitation, to the peak area in absence of matrix.
The stability of the three analytes in serum was evaluated at two concentrations, low (1 ng mL–1 of AP and AP7G, and 5 ng mL–1 of CA) and high (50 ng mL–1 of AP, AP7G and 100 ng mL–1 of CA), in three replications for each concentration. Stability was checked after three freeze–thaw cycles at − 20 °C and long-term storage (2 months). For short-term stability, samples before processing were stored on a benchtop for 2 h at room temperature. Furthermore, stability in samples after processing was also assessed after storage in an autosampler for 24 h. The concentrations of the analytes after each storage period were calculated using a calibration curve, obtained from freshly prepared samples in the same analytical run. Based on the FDA guidelines of bioanalytical method validation, the stability of the analytes is confirmed if the deviation from the nominal concentration is within ± 15% [18].
The Application of UPLC–MS/MS for Analysis of the Studied Compounds in Goats
Blood serum from sixty clinically healthy Polish white improved dairy goats was analyzed. The goats were kept on a specialised farm located in northwestern Poland (Bukowiec, 52°5104100 N; 16°5201200 E) in the Wielkopolska region. The animals were aged 20–30 months, in their second lactation, and had a body weight of 56–60 kg. The goats diet were formulated to meet the animals’ nutrient requirements: 2.12 UFM (unit for milk production) and 185 g PDI (protein truly digestible in the small intestine) to obtain an assumed milk yield of 3.0 kg and 3.8% of fat [19]. The ingredients % DM (dry matter) of diet offered to dairy goats were: 15.6% maize silage; 21.6% grass hay silage; 7.8% brewers’ grain silage; 37% concentrate mixture; 10.3% meadow hay; 4.4% dried sugar beet pulp; and 3.3% barley straw.
All 60 goats used in the feeding experiment were divided into 2 groups: 30 groups were fed the standard diet as mentioned above (Control group) and 30 goats additionally received a supplement containing nine herbs in the daily dose 40 g/day to the received feed (Feeding group with herbal mixture) according to our previous work [8]. The herbs included were common nettle Urtica dioica L. (herb); common agrimony Agrimonia eupatoria (herb—dried flowering shoot tips); coriander Coriandrum sativum (fruit); fenugreek Trigonella foenum graecum L. (seeds), as well as fennel Foeniculum vulgare (fruit); peppermint Mentha piperita (leaves); chamomile Matricaria chamomilla L. (flower clusters); milk thistle Silybum marianum (endosperm); and thyme Thymus vulgaris (leaves) [8]. A detailed contribution of particular herbal components included in the supplement is presented in our patent applications (Polish Patent Office submissions P.4334426 and P.433779).
The blood was collected in the middle of lactation, when goats were approximately 138.1 ± 2.7 days in milk (DIM). The blood was collected from the jugular vein to 10 mL dry tubes. The tubes were left for 8 h at room temperature to allow for clot formation, then, centrifuged at 3000 rpm for 10 min and the serum was stored in 2 mL Eppendorf vials at − 20 °C until being analyzed using the validated UPLC-MS/MS method. All animal procedures were approved by the Local Ethical Committee for Animal Research (Poznań, Poland; decision no. 57/2020).
Statistical Analysis
Statistica 13.3 (TIBCO Software Inc., Tulsa, OK, USA) was used to carry out the statistical assessment. Shapiro–Wilk test was used to assess the normality of AP, AP7G and CA concentrations in the studied groups. The analyte concentrations were represented as mean and standard deviation (SD). Student’s t test or Mann–Whitney test were applied to compare the differences in concentrations between two goat groups. The p value < 0.05 was considered statistically significant for the test.
Results and Discussion
UPLC-MS/MS Analysis
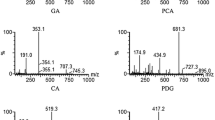
MS conditions have been adjusted to obtain a high sensitivity for AP, AP7G, CA, and APd5. The protonated molecular ions [M + H]+ were chosen as precursor ions for the MS/MS fragmentation analysis of the analytes. The parent ion of AP7G with m/z 432.8 Da undergoes fragmentation primarily by loss of the glucose residue (~ 162 Da) resulting in the aglycon ion, AP with m/z 271 Da. Other daughter ions of AP7G, including m/z 153 and 119 Da, result from fragmentation of the AP structure. The mass fragmentation spectra of the studied compounds with possible chemical structures of the ions are presented in Fig. 2.
The column, composition of the mobile phase, and gradient elution have been chosen to get all analytes separated and to display a good resolution for their peaks in a retention time of 10 min, which is relatively short. Chromatographs of the blank serum, serum spiked with the standard solution, and a real sample from a goat are displayed in Fig. 3.
For the isolation of polyphenols from animal biological matrices, various methods were applied based mainly on liquid–liquid extraction [17], solid phase extraction [15] and protein precipitation [9,10,11,12,13,14, 16]. In our method, simple protein precipitation with acetonitrile was applied with further solvent evaporation and reconstitution in methanol:water mobile phase. During the method development, acetonitrile was proven to be more efficient than methanol for serum protein precipitation which was also reported by Polson et al. [20]. The present method requires 200 µL of serum, more than 100 µL reported by other authors [9,10,11,12,13]. However, it allowed us to obtain a higher sensitivity of the measurements compared to these reports, as discussed in Sect. “Method Validation”.
Method Validation
Detection of AP, AP7G and CA using MRM mode was highly selective, with no interferences from endogenous compounds at the expected retention time of the analytes in blank plasma samples obtained from six individual goats. No significant carry-over was noticed for the compounds. The standard curves of AP, AP7G and CA were linear in the concentration range: 0.5–50 ng mL–1 for AP and AP7G, and 2.5–100 ng mL–1 for CA, which was confirmed by the correlation coefficient r values ≥ 0.998 for all compounds. Calibration curves’ equations, correlation coefficients, and linear ranges are presented in Table 3.
Under the stated experimental conditions, LLOQ was 0.5 ng mL–1 for AP and AP7G, and 2.5 ng mL–1 for CA. The current LLOQ value for AP is lower than the values obtained in rat serum by other authors: 1 ng mL–1 [10, 12], 1.77 ng mL–1 [10], 2.5 ng mL–1 [13]. LLOQ of CA was 2.5 ng mL–1, which is lower than the value of 10 ng mL–1 received by Sun et al. [11]. Jia et al. [9] reported LLOQ \(\le\) 0.2 ng mL–1 for AP, AP7G and CA but the method was linear in the range 5–1000 ng mL–1 for all three compounds. The inter- and intra-day precision of the method expressed as %RSD was in the range of 1.22–14.7%. Accuracy of the method expressed as relative error (%RE) was in the range of 0.147–14.6% (Table 4). The results of the accuracy and precision were within the FDA recommendation for the method validation [18].
Recoveries of the analytes from goat serum following protein precipitation were between 76.2 for CA and 89.5% for AP (Table 5) and were similar to the values reported in literature data for rat plasma [9,10,11,12, 14]. A lower recovery (61–76%) of polyphenols was reported by Cheruvu et al. [17] when using liquid–liquid extraction with ethyl ether for mice plasma. The matrix effects expressed by MF ranged between 0.87 and 1.10 for all analytes. %RSD of MF was below 15% regardless of the level of concentration measured (Table 5). These results indicated that both the matrix effect and extraction recovery are satisfactory.
Stability results showed that AP, AP7G and CA were stable in goat serum for at least 2 months at − 20 °C and after three freeze–thaw cycles, which was confirmed by the relative error (%RE) estimated in the ranges of 1.80–12.0% and 2.80–11.6% respectively. All the analytes were stable in samples before processing when stored for 2 h at room temperature (%RE: 1.80–13.0%). The stability test results for the analytes in processed samples while stored in the autosampler for 24 h were within the %RE range of 8.5–15.0% (Table 6).
The good stability of the compounds in rat plasma was also confirmed by other authors [9,10,11,12]. Jia et al. [9] reported that AP, AP7G and CA were stable when stored in autosampler for 12 h, at room temperature for 6 h, at − 80 °C for 14 days, and through three freeze/thaw cycles. However, according to Sun et al. [11], CA had lower stability at room temperature (2 h) and at − 70 °C (7 days).
In vivo Study
The validated UPLC–MS/MS method was successfully applied for the analysis of AP, AP7G and CA in all 60 samples of goat serum. The determined concentrations of AP, AP7D and CA were in the ranges of 0.50–5.23 ng mL–1 (mean value 1.14 \(\pm\) 0.16 ng mL–1), 0.50–6.75 ng mL–1 (mean value 0.89 \(\pm\) 0.15 ng mL–1) and 2.50–45.1 ng mL–1 (mean value 4.97 \(\pm\) 0.93 ng mL–1), respectively. Detailed distribution of the concentrations of the above-mentioned both analyzed groups is presented in Fig. 4. It was found that the animals fed with fodder additionally containing the mixture of herbs (Feeding group with herbal mixture) had significantly (p < 0.001) higher concentrations of all tested compounds in relation to the traditionally fed group (Control group), namely: AP: 1.72 ± 0.29 vs. 0.56 ± 0.02 ng mL–1; AP7G: 1.23 ± 0.29 vs. 0.56 ± 0.05 ng mL–1; CA: 7.33 ± 1.76 vs. 2.61 ± 0.11 ng mL–1 (Fig. 4).
Until now, there is no literature data on the AP, AP7G and CA pharmacokinetics in goats. There are several reports which show that concentrations of the compounds determined in rat plasma show high differences depending on the plant extract given orally to the animals. Jia et al. [9] reported the following maximum concentrations (Cmax) of the compounds after oral administration of 10 g kg–1 of Flos Chysantemi extract: 301.8 ± 87.1 ng mL–1 of AP, 96.4 ± 24.8 ng mL–1 of AP7G, 194.4 ± 56.4 ng mL–1 of CA. However, lower values of AP and CA in rat plasma were obtained by other authors. Intragastric injection of Buddleja lindleyana Fort. extract resulted in AP levels of 146.4 ± 14.9 ng mL–1 [10] while after oral administration of Semen cuscutae extract Cmax of CA was 35.5 ± 7.30 ng mL–1 [12].
Relatively low concentrations of the analyzed compounds in the goat serum result from the administration of the herbal mixture as an additive to the feed received by the animals and, as already mentioned, the unknown degree of absorption (bioavailability) of these substances from the gastrointestinal tract in goats. However, it should be emphasized that despite the low concentrations obtained, the overall nutritional effect in the experiment was very positive, significantly affecting the health of goats, which was demonstrated in our previous work on the effect of herbal feed additive on lactic acid bacteria count in the goats [8]. Therefore, clearly higher concentrations of the tested compounds in the group fed with herbs may in this case serve as indicators of the mixture used to improve the health of goats.
Conclusions
A reliable and sensitive UPLC-MS/MS method was developed to measure concentrations of AP, AP7G and CA in goat serum. The method is precise and accurate and fulfils the validation requirements for stability, recovery and matrix effect regarding quantitative analysis of bioactive compounds in biological fluids. The validated UPLC-MS/MS method was successfully applied for determination of the analyte concentrations in serum samples of sixty goats. The observed higher concentrations of AP, AP7G and CA in serum of the animals fed with fodder supplemented with herbal mixture pointed to the positive nutritional effect which may improve the goat health and quality of dairy products.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Madunić J, Madunić IV, Gajski G et al (2018) Apigenin: a dietary flavonoid with diverse anticancer properties. Cancer Lett 413:11–22. https://doi.org/10.1016/j.canlet.2017.10.041
Salehi B, Venditti A, Sharifi-Rad M et al (2019) The therapeutic potential of apigenin. IJMS 20:1305. https://doi.org/10.3390/ijms20061305
DeRango-Adem EF, Blay J (2021) Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front Pharmacol 12:681477. https://doi.org/10.3389/fphar.2021.681477
Lu X-Y, Sun D-L, Chen Z-J et al (2010) Relative contribution of small and large intestine to deglycosylation and absorption of flavonoids from Chrysanthemun morifolium extract. J Agric Food Chem 58:10661–10667. https://doi.org/10.1021/jf102992r
Wang L, Pan X, Jiang L et al (2022) The biological activity mechanism of chlorogenic acid and its applications in food industry: a review. Front Nutr 9:943911. https://doi.org/10.3389/fnut.2022.943911
Clifford MN, Kerimi A, Williamson G (2020) Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Comprehensive Rev Food Sci Food Safety 19:1299–1352. https://doi.org/10.1111/1541-4337.12518
Gonthier M-P, Verny M-A, Besson C et al (2003) Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J Nutr 133:1853–1859. https://doi.org/10.1093/jn/133.6.1853
Foksowicz-Flaczyk J, Wójtowski JA, Danków R et al (2022) The effect of herbal feed additives in the diet of dairy goats on intestinal lactic acid bacteria (LAB) count. Animals 12:255. https://doi.org/10.3390/ani12030255
Jia Q, Huang X, Yao G et al (2020) Pharmacokinetic study of thirteen ingredients after the oral administration of Flos Chrysanthemi extract in rats by UPLC-MS/MS. Biomed Res Int 2020:1–10. https://doi.org/10.1155/2020/8420409
Zhang X, Zhang Z, Zhang L et al (2021) The development and validation of a sensitive HPLC-MS/MS method for the quantitative and pharmacokinetic study of the seven components of Buddleja lindleyana Fort. RSC Adv 11:26016–26028. https://doi.org/10.1039/D1RA04154A
Sun M, Yin Y, Wei J et al (2018) Development and validation of a HPLC-MS/MS method for simultaneous determination of twelve bioactive compounds in epimedium: application to a pharmacokinetic study in rats. Molecules 23:1322. https://doi.org/10.3390/molecules23061322
Shen J, Jia Q, Huang X et al (2019) Development of a HPLC-MS/MS method to determine the 13 elements of Semen Cuscutae and application to a pharmacokinetic study in rats. Evidence-Based Complementary Alternative Med 2019:1–11. https://doi.org/10.1155/2019/6518528
Wan L, Guo C, Yu Q et al (2007) Quantitative determination of apigenin and its metabolism in rat plasma after intravenous bolus administration by HPLC coupled with tandem mass spectrometry. J Chromatogr B 855:286–289. https://doi.org/10.1016/j.jchromb.2007.05.007
Zhu S, Zhang J, Lv Z, Yu M (2021) LC-MS/MS determination of apigenin in rat plasma and application to pharmacokinetic study. CPB 22:274–280. https://doi.org/10.2174/1389201021666200807113144
Hanske L, Loh G, Sczesny S et al (2009) The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J Nutr 139:1095–1102. https://doi.org/10.3945/jn.108.102814
Cai H, Raynaud D, Steward WP, Gescher AJ (2006) A simple HPLC method for the determination of apigenin in mouse tissues. Biomed Chromatogr 20:1038–1042. https://doi.org/10.1002/bmc.634
Cheruvu HS, Yadav NK, Valicherla GR et al (2018) LC-MS/MS method for the simultaneous quantification of luteolin, wedelolactone and apigenin in mice plasma using hansen solubility parameters for liquid-liquid extraction: application to pharmacokinetics of Eclipta alba chloroform fraction. J Chromatogr B 1081–1082:76–86. https://doi.org/10.1016/j.jchromb.2018.01.035
Guideline on bioanalytical method validation—guideline-bioanalytical-method-validation_en.pdf. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Accessed 12 Oct 2019
Bagnicka E, Wojtowski J, Uniwersytet Przyrodniczy (Poznan), Wydawnictwo (2021) Hodowla, chow i uzytkowanie koz. Wydawnictwo Uniwersytetu Przyrodniczego, Poznan
Polson C, Sarkar P, Incledon B et al (2003) Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography–tandem mass spectrometry. J Chromatogr B 785:263–275. https://doi.org/10.1016/S1570-0232(02)00914-5
Funding
This research was funded by NATIONAL CENTRE for RESEARCH and DEVELOPMENT (NCBiR), grant number POIR.04.01.02-00-0008/18.
Author information
Authors and Affiliations
Contributions
MK-Ł: methodology, validation, investigation, writing—original draft, visualization. JAW: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—review and editing. FG: writing—review and editing, supervision. RD: investigation, formal analysis, resources, project administration. JP: formal analysis, project administration, AG: investigation, resources. JF-F: conceptualization, methodology, validation, formal analysis, investigation, resources, writing—review and editing. PŁM: methodology, validation, investigation, writing—original draft, visualization, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karaźniewicz-Łada, M., Wójtowski, J.A., Główka, F. et al. Application of UPLC-MS/MS Method for Analysis of Apigenin, Apigenin 7-Glucoside and Chlorogenic Acid in Goat Serum. Chromatographia 86, 401–411 (2023). https://doi.org/10.1007/s10337-023-04250-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04250-7