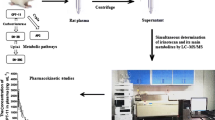
Abstract
Combination therapy of gemcitabine and sorafenib is synergistically effective and well tolerated in patients with non-small cell lung cancer (NSCLC). In this study, the pharmacokinetic parameters of both gemcitabine and sorafenib were estimated after intra-peritoneal administration in rats using novel, green and sensitive RP-HPLC and TLC-densitometric methods where sildenafil used as an internal standard. Firstly, the developed RP-HPLC method achieved on ZORBAX Eclipse Plus C18 (4.6 mm × 150 mm, 5 μm) using a mixture of methanol: 40 mM ammonium acetate solution. On the other hand, the developed TLC-densitometric method was achieved on TLC plates using a developing system of methanol: ethyl acetate (3: 7, by volume) and scanning wave length at 260 nm. Additionally, the developed methods were validated according to FDA guidelines. Moreover, the developed methods were successfully used to study the pharmacokinetic parameters of both gemcitabine and sorafenib after administration of each drug alone and co-administration in rats. The results presented that drug–drug interaction between drugs happened when administrated together affecting the pharmacokinetic parameters of each other. The proposed methods, being ecofriendly, accurate, and sensitive, may become the corner stone for further clinical studies of the studied drugs determinations applied on humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer (LC) is a leading cause of high mortality which responsible for nearly 1.8 million deaths every year. Non-small cell lung cancer (NSCLC) is a major type of lung cancer that accounts for 80–85% [1]. There are different types of chemotherapeutic regimens that used for treatment of NSCLC. However, the rate of survival and recovery are still very low [2].
FDA is trying to find suitable regimen by combination between two or more chemotherapeutic agents which helps in improving the synergistic therapeutic efficacy as well, decreasing adverse effects related to high-dose drugs [3].
Gemcitabine (GEM) is a pyrimidine nucleoside, chemical name 4-amino-1-[(2R, 4R, 5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)-oxolan-2-yl]pyrimidin-2-one monohydrochloride (Fig. 1a) that has been approved as first-line treatment for NSCLC for long time, but harmful side effects and recovery resistance have been found with gemcitabine therapy [4].
Sorafenib (SOR) is a non-selective multi-kinase inhibitor, chemical name 4-(4-{3-[4-chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)-N2-methylpyridine-2-carboxamide-4-methylbenzenesulfonate (Fig. 1b). Preclinical studies of the efficiency of sorafenib in treatment of NSCLC models have been reported [5]. On the other hand, there are limitations in dose of sorafenib due to its adverse side effects [6].
Recently, there is a novel combination strategy of gemcitabine with sorafenib which synergistically interacts to NSCLC cells showing cytotoxicity and anti-invasive effects. Furthermore, this combination has been found to be well tolerated in patients with NSCLC [7, 8].
Literature review has revealed some analytical methods for determination of GEM alone and in combinations with other drugs from different biological samples by spectrophotometric method [9], high-performance liquid chromatography (HPLC) [10,11,12,13] and liquid chromatography–tandem mass spectrometry (LCMS/MS) methods [14,15,16].
On the other hand, SOR was determined alone and in combinations with other drugs from biomatrices by spectrophotometric method [17], thin-layer chromatography (TLC) [18], HPLC methods [19, 20] and LCMS/MS methods [21, 22].
Efficacy and safety of the chemotherapeutic combination should be confirmed by studying the pharmacokinetics of drug. Till now, there was no analytical method for determination of the GEM and SOR simultaneously from plasma matrix for studying the pharmacokinetic profile of single and combined doses in vivo.
One of our challenges is to find benign analytical method using green chemistry and environment-friendly substances for separation and determination the studied compounds without using any hazardous agents during the whole analytical procedure. Currently, estimation of the greenness of analytical method can be achieved using different tools as eco-scale assessment [23], analytical greenness metric approach (AGREE) [24], green analytical procedure index (GAPI) [25] and national environmental method index (NEMI) [26] which can be used to evaluate the greenness profile of any analytical experiment.
Our purpose in the present study is concerned to develop novel RP-HPLC and TLC- densitometric methods for concurrent evaluation of GEM and SOR in rat plasma alone and in combination to study the in vivo pharmacokinetic drug–drug interaction in rats with advantages of being sensitive, accurate and rapid methods to be suitable for routine clinical examination. Taking into account the environmental aspects, the developed methods were achieved without using any harmful substance like acetonitrile in RP-HPLC method or toluene and chloroform in TLC- densitometric method.
Materials and methods
Instruments
- For plasma samples preparation:—20 to + 40 °C Electrical Centrifuge with 800—15,000 rpm (Hettich, Germany) was used for centrifugation of samples, it has a capacity of 32 × 15 mL with a voltage of 200—240 V and 300 VM vortex mixer (Gemmy Industrial Corp, Taiwan) was also used.
-For RP-HPLC method: (Agilent 1260 Infinity Quaternary HPLC) was used that was equipped with an autosampler, and a diode array detector using a reversed phase stationary phase column ZORBAX Eclipse Plus C18 (4.6 mm × 150 mm, 5 μm). All data and signals were processed using Agilent control software.
-For TLC-densitometric method: (CAMAG, Muttenz, Switzerland) which consist of densitometric TLC Scanner 3 controlled by Win CATS software (Version 1.2.0), deuterium and tungsten lamp was the source of radiation, CAMAG sample applicator (Linomat V) equipped with a 100 μL syringe and CAMAG twin through glass tank. UV lamp (VL-6.LC; Marne La Vallee, France) with 254 nm short wavelength was used during method optimization. TLC plates (Merck, Darmstadt, Germany) coated with 60 F254 silica gel (20 × 10 cm), 0.2 mm thickness was used as stationary phase.
Materials and reagents
Pure samples
Gemcitabine and sorafenib were purchased from Sigma-Aldrich CO., Cairo, Egypt. Their purity were estimated using the developed methods and was found to be100.93% and 100.99%, respectively.
Sildenafil (internal standard) was supplied by EIPICO CO., El Horria St., Zone B 10th of Ramadan City, Egypt with purity of 99.32% according to manufacturer certificate of analysis.
Chemical and solvents
All reagents used during those methods were of analytical grade. Methanol, ethanol and ethyl acetate were obtained from El NASR Pharmaceutical Chemicals Co., Gesr El Suez, Cairo, Egypt. Methanol and 0.25 M liquid ammonium acetate of HPLC grade were obtained from Sigma-Aldrich CO., Cairo, Egypt. Deionized water was purchased from SEDICO Pharmaceuticals Co., 6th October City, Egypt.
Chromatographic conditions
RP-HPLC method
RP-HPLC method was achieved on ZORBAX Eclipse Plus C18 (4.6 mm × 150 mm, 5 μm) by using gradient elution and mobile phase consisted of methanol: 40 mM ammonium acetate solution (pH = 5.5) at a flow rate of 1.3 mL/min, the injection volume was 20 μL, temperature was adjusted at 25 °C and the run time was 8 min using UV detection at 260 nm.
TLC-densitometric method
TLC- densitometric method was carried out by applying sample drugs and internal standard (SIL) spots to TLC plates using CAMAG Linomat V applicator. Ascending development was achieved in a twin-trough chamber that was previously saturated for 15 min with developing system consisting of ethyl acetate:methanol (7: 3, by volume) at room temperature using UV lamp with wavelength of 260 nm.
Prepared solutions
Stock standard solutions of GEM, SOR, and SIL were prepared in concentration of 1000 μg/mL by precisely weighing 0.1 gm of each compound and transferred into three separate 100-mL volumetric flasks and dissolving in 75 mL methanol HPLC. Then, the volume was adjusted to the mark with the same solvent. Additionally, working standard solutions were prepared by diluting the stock solutions with the mobile phase mixture for RP-HPLC and with methanol for TLC-densitometric method to obtain concentration of 10 μg/mL of each GEM and SOR and 100 μg/mL of IS (SIL).
Procedure
Construction of calibration curves
For RP-HPLC method
Different concentrations of GEM and SOR in the range of 0.03–30 μg/mL were prepared from their working standard solutions (10 μg/mL) and stock standard (1000 μg/mL) for each drug. Those different concentrations were spiked with 0.5 mL of drug free rat plasma previously treated with tetrahydrouridine (40 µL of 10 mg/mL THU to 5.0 mL of plasma), an inhibitor of cytidine deaminase to prevent metabolism of GEM to 2', 2' difluorodeoxyuridine (dFdU) and then 0.25 mL of IS (SIL) added from its working solution (100 μg/mL). Additionally, the volume was adjusted to 5 mL with methanol, and then plasma protein was precipitated. The prepared solutions were mixed well using vortex for two minutes and then centrifuged at 4000 rpm for 10 min at 2 °C.
The clear supernatant was transferred and accurate volume of 20 μL of each sample was injected automatically by auto-sampler. Then, the obtained peak-area ratios of each drug (relative to the internal standard) were constructed against its corresponding concentrations relating calibration curves and regression equations were calculated.
For TLC-densitometric method
Different concentrations of GEM and SOR in the range of 1.2–800 μg/mL and 1.2–160 μg/mL, respectively were prepared from their working standard solutions (10 μg/mL) and stock standard (1000 μg/mL) for each drug, then spiking those different concentrations with 0.5 mL of drug free rat plasma previously treated with THU and 0.1 mL of IS (SIL) added from its stock standard solution (1000 μg/mL). After that, the volume was adjusted to 5 mL with methanol and plasma protein was precipitated. The prepared solutions were mixed well using vortex for 1 min and then centrifuged at 4000 rpm for 10 min at 2 °C.
The clear supernatant was transferred and accurate volume of 25 μL of each sample was applied on TLC plates to obtain concentrations in the range of 0.03–20 µg/band and 0.03–4 µg/band for GEM and SOR, respectively.
Moreover, the obtained peak-area ratios of each drug (relative to the internal standard) were constructed against its corresponding concentrations relating calibration curves and regression equations were calculated.
Treatment of animals and aggregation of plasma samples
All animal research was achieved in accordance with the Ethics Committee for Animal Experimentation (Institutional Animal Care and Use Committee, Beni-Suef University) with approval number: 022–255 and guidelines of the Guide for the Care and Use of Laboratory Animals [27].
28 adult male Wistar rats (average weight 250 g) were housed for 5 days prior to treatment under standard laboratory conditions of temperature (22–25 °C) and light cycle (12–14 h per day) in a well-air renewed place.
They were randomly divided into four groups (n = seven for each group) and the studied drugs were taken through intra-peritoneal route. Group I–III were the treated groups with doses of 130 mg/kg dose of GEM (group I), 60 mg/kg dose of SOR (group II) and combined doses of 130 mg/kg GEM and 60 mg/kg SOR (group III). Finally group (IV) was used as the control group that did not receive any drug. GEM and SOR were dissolved in saline solution and were administered by intra-peritoneal injection (130 mg/kg, 60 mg/ kg body weight, respectively).
Furthermore, 1 mL blood sample from each rat was taken from retro-orbital plexus at different time intervals (0.25, 0.5, 1, 3, 6, 8, and 24 h) following drug administration. Blood samples were collected in THU-pretreated heparinized tubes, then immediately plasma was separated from blood samples by centrifugation at 4000 rpm for 20 min and then stored at − 20 °C till analysis time.
Preparation of the collected plasma samples
A 0.5 mL of previously collected frozen plasma samples were separately transferred into tubes after thawing to room temperature and then spiked with a constant volume of IS (SIL) of 50 μL for RP-HPLC and 200 μL for TLC-densitometric from its working solution (100 μg/mL). The volume of each was completed to 1 mL with methanol, after that samples were mixed by vortex for 2 min and centrifuged for 10 min at 4000 rpm. Finally, 20 μL and 25 μL of the clear supernatant was then applied for RP-HPLC and TLC-densitometric methods, respectively and analyzed according to the instructions of each of the developed methods.
Method validation
Validation of the methods was achieved to estimate the linearity, accuracy, precision sensitivity, selectivity and matrix effect. Validation was performed according to the recommendations of FDA guidelines [28].
Linearity, calibration curves and limits of quantification
Calibration curves were constructed in the range of 0.03–30 μg/mL of each drug for RP-HPLC method, 0.03–20 μg/band of GEM and 0.03–4 μg/band of SOR for TLC-densitometric method by relating the peak-area ratio of each compound (relative to the internal standard) against its corresponding concentrations and regression equations were calculated.
Lower limit of quantification (LLOQ)
It was the lowest and first concentration of the calibration curve (0.03 μg/mL of both GEM and SOR for RP-HPLC and 0.03 μg/band TLC-densitometric methods) at which the drug response should be at least five times as the response of blank plasma and its response should have precision that not exceed 20% of coefficient of variation (CV = SD/mean) or relative standard deviation (RSD).
Upper limit of quantification (ULOQ)
It was the highest and final concentration of the calibration curve (30 μg/mL of both GEM and SOR for RP-HPLC, 20 μg/band of GEM, 4 μg/band of SOR for TLC-densitometric method) that have a precision not exceed 15% of coefficient of variation or relative standard deviation and have accuracy within 100 ± 15% of the true concentration.
Quality control samples (QCs)
Three QCs were selected for method validation following the same procedure shown under instruction of calibration curves.
There are different concentrations of GEM and SOR were prepared to cover the three ranges of QC samples: low quality control (LQC), middle quality control (MQC) and high quality control (HQC) for each method.
Firstly, LQC, MQC and HQC in RP-HPLC method were 0.08, 1 and 10 μg/mL, respectively for GEM and SOR. On the other hand, in TLC-densitometric method were 0.04, 0.2 and 10 μg/band for GEM while 0.04, 0.2 and 2 μg/band for SOR, respectively. All the prepared samples were directly stored at − 20 °C till the time of analysis.
Accuracy
Estimation of accuracy of the developed methods was achieved by using quality control samples according to the procedure under linearity. The concentration of GEM and SOR were calculated from the computed regression equations which % recoveries should within ± 15% for QCs and 20% for LLOQ.
Precision
Repeatability (intra-day precision) was estimated by analyzing of QCs in triplicates within the same day while intermediate (inter-day) precision was determined by repeating the analysis of QCs on three successive days using previously procedure under the instruction of each method and then % RSD was calculated which should ≤ 15%.
Selectivity
Selectivity was evaluated by the ability of the developed methods to resolve the compounds from plasma matrix through comparing the chromatograms of the blank plasma with QCs of the studied drugs to estimate endogenous interferences.
Extraction recovery and matrix effect
The matrix effect was tested to confirm that there is no effect of the plasma matrix on the recoveries of the studied drugs which achieved by comparing the peak area ratio obtained from QC samples (spiked pure drugs with plasma) with those of pure standards solutions having same concentrations.
Stability
Stability of the compounds in the plasma matrix was checked by exposing QCs to three different conditions such as room temperature (25 °C) for 6 h (Bench-Top stability), 24 h at auto-sampler (Post Preparative stability) and three freeze–thaw cycles by thawing from − 20 °C to room temperature [freeze and thaw stability]. Then, mean percentage recoveries were calculated which should be 100 ± 15%.
Application to pharmacokinetic study
The developed methods were performed to estimate pharmacokinetic parameters of GEM and SOR when administered alone and in combination. Then, curves were constructed relating plasma concentration of GEM and SOR when administered alone and in combination against time. Pharmacokinetic parameters were evaluated as clearance (Cl), apparent volume of distribution (Vd), elimination rate constant (k) and half-life (t1/2). Finally, analysis of variance (ANOVA) test was used to test the difference between groups.
Results and discussion
Knowledge of a compound’s pharmacokinetics (PK) and any potential drug-drug interactions is paramount and is demonstrated by requirements by regulatory authorities world-wide. It is, therefore, not surprising that determination of a drug’s PK forms an important part of clinical trials especially in patients who suffering from cancer disease and could not tolerate the adverse drug reaction related to concentration of each drug in their treatment protocol.
Our aim was to develop simple, green and sensitive RP-HPLC and TLC-densitometric methods to study the effect of each of GEM and SOR on the concentration of the other one via this in vivo study to assure that effect.
Method optimization
RP-HPLC method
A validated RP-HPLC method was established to provide green, reliable and rapid method for simultaneous determination of GEM and SOR. To optimize the developed RP-HPLC method, different parameters were tested that affect the efficiency of separation.
Firstly, different stationary phases were tried including C18 (4.6 mm × 100 mm, 3.5 μm), but unresolved peaks and poor separation between GEM, SOR and IS were obtained due to short column length. Then, other columns with longer length were tested such as: C18 (4.6 mm × 150 mm, 5 μm) and SB-Phenyl (4.6 mm × 150 mm, 1.8 μm) columns. Finding that column of C18 (4.6 mm × 150 mm, 5 μm) showed good resolution between all compounds.
Furthermore, several mobile phases were tested having different polarities and compositions depending on utilizing green and ecofriendly solvents and exclusion of harmful ones. Firstly, ethanol:water with different ratios was tried resulting in unresolved separation between drugs. Then, ethanol was replaced with methanol trying to resolve overlapping peaks, but not enough separation was resulted between GEM and plasma peaks. So, pH modification was tested with different acidic and basic pH as formic acid, triethylamine and ammonium acetate. It was found that 40 mM ammonium acetate buffer (pH = 5.5) resolved the overlapping peaks between GEM and plasma at ratio of methanol: 40 mM ammonium acetate (15:85 v/v).
On the other hand, different flow rates (0.8, 1, and 1.3 mL/min) were also tried to separate the studied compounds within suitable analysis run time and finding that the best flow rate was 1.3 mL/min.
Isocratic elution using this ratio did not work well since SOR and IS retained on column and required high ratio of non-polar solvent to elute them. Hence, gradient elution was the choice and successful to resolve the studied drugs and plasma. Finally, gradient program of methanol: 40 mM ammonium acetate as shown in Table 1 was performed and resulting in good separation between drugs at reasonable time.
As well, different scanning wavelengths (220, 254, 260 and 268 nm) were tested. UV spectrum of the studied drugs revealed different λ max of each one which were 268 nm, 264 nm and 295 nm for GEM, SOR and IS, respectively in addition great absorbance for each one of them at a wide range of wavelengths (220─300). The wavelength of 260 nm was found to be the optimum one to provide a good sensitivity for the three studied drugs. Using of the most suitable and selected chromatographic conditions, resulting in the best separation of the studied compounds with reasonable retention times 1.933 min, 5.439 min and 6.946 min for GEM, SIL and SOR, respectively (Fig. 2).
TLC-densitometric method
Our main goal was to obtain the best resolution between the separated components using simple and green mobile phases. So, different mobile phases were tested, firstly (methanol:methylene chloride) in different ratios, but it was not sufficient.
Moreover, we turned to more polar solvent using mixture of (methanol:ethyl acetate).
The first tested ratio was (5:5 v/v), but GEM and SOR traveled faster with the front line without any separation. Good separation between those tested components was reached upon using (methanol:ethyl acetate in ratio 3:7 v/v) this increasing in ethyl acetate made the desired separation with good resolution between the studied drugs. Good symmetric peaks were obtained at Rf values 0.39, 0.69 and 0.88 for SIL, GEM and SOR, respectively (Fig. 3).
To assure the sensitivity of the proposed methods, different UV scanning wavelengths were tested (220, 254, 260 and 268 nm). It was found that 260 nm wavelength showed the best result. Additionally, saturation time was tested and 15 min was found to be the optimum one.
Method validation
Linearity and calibration curves
Linear calibration curves were obtained in the ranges of 0.03–30 μg/mL of both GEM and SOR for RP-HPLC method, while in the ranges of 0.03–20 μg/band of GEM and 0.03–4 μg/band of SOR for TLC-densitometric method. Regression data showed high and satisfactory correlation coefficients; 0.9997 and 0.9998 for both components in the proposed methods. Regression parameters are shown in Table 2.
Accuracy and precision
Accuracy was evaluated and values of % recoveries were acceptable (100 ± 15%). Also, intra- and inter-day precision were evaluated and values of % RSD were satisfactory in the range of 2.55 to 5.69% and 3.52 to 6.75% for GEM and SOR, respectively in RP-HPLC method while in TLC-densitometric method it was ranged from 3.08 to 7.02% and 2.36 to 6.34% for GEM and SOR, respectively. Results presented in Table 3 indicate that the developed methods are precise and reproducible for quantitative determination of the studied compounds simultaneously.
Selectivity
There is no interference from endogenous plasma constituents with the studied drugs when compared blank plasma with plasma spiked with GEM, SOR and SIL shown in (Figs. 2 and 3).
Extraction recovery and matrix effect
Extraction recovery of GEM and SOR was evaluated and it was ranged from 90.37 to 102.64% for GEM and from 94.88 to 104.15% for SOR in RP-HPLC while in TLC-densitometric method it was ranged from 89.52 to 103.46 and 89.04 to 97.57 for GEM and SOR, respectively. These results showed the efficiency of the extraction method and confirmed that there was no significant effect of plasma matrix on the extraction of the compounds (Table 4).
Stability
Studying the stability of QCs using different conditions showed that no significant effect of those conditions on plasma samples confirming that samples concentrations were stable (Table 5).
Pharmacokinetic study
Estimation of the pharmacokinetic parameters of GEM and SOR were achieved by the developed RP-HPLC and TLC-densitometric methods when administered to rats either alone or in combination through intra-peritoneal route. The collected plasma samples at the selected time interval were prepared and extracted readily for analysis using the proposed methods as previously mentioned. The concentration of each sample was calculated using the regression equation then plotted against its relative time interval (Fig. 4).
Different pharmacokinetic parameters were calculated as elimination rate constant (K), volume of distribution (Vd), clearance (Cl), Cmax, half-life t1/2, tmax, AUC all and infinity as shown in Table 6. ANOVA test on the pharmacokinetic parameters and Newman–Keuls Multiple Comparison test were done to detect the significant differences between groups in Tables 7 and 8, respectively showing that there was a significant increase in concentration of GEM and SOR when administered in combination comparing with their respective values of concentrations when each one was administered alone.
Our data suggest an interaction between GEM and SOR as likely, so taking those drugs together must be with certain considerations concerning the dose calculation for co-administered protocols which may improve the efficacy and may limit some adverse effects related to increasing concentration. On the other hand, some difference may be found in its results when applying on human as this study achieved on rats.
Evaluation of the greenness of the developed methods
Analytical eco-scale of the developed methods
It is known as semi-quantitative approach to evaluate the greenness of analytical methods [23]. The analytical Eco-Scale is estimated by calculation of giving penalty points to all parameters of those methods that opposes with green analysis which calculated as follows: Analytical Eco-Scale score = 100—total penalty. The higher Eco-Scale value obtained the more ideal and green analysis method achieved.
The greenness of the method is counted to be excellent, if the score > 75 and to be acceptable if the score > 50 [23]. According to Eco-Scale approach, penalty points reflect the harmless properties of the method which depend on various parameters including consumption of toxic solvents, chemicals, energy, occupational hazards and production of waste.
Both RP-HPLC method and TLC-densitometric method had Eco-Scale score > 75 as presented in Table 9, so the developed methods are considered to be ecofriendly ones and can be safely used for monitoring of GEM and SOR with low hazards on environment and human health.
Analytical greenness metric approach
The AGREE [24] is one tool for estimation the greenness of the suggested methods which based on assigning a score to each of the twelve principles of green analytical chemistry where the total score is a fraction of one, ranging from zero to one. Its basic principles concerned with different parameters such as simplicity of the method, waste production, and energy consumption. The total score for the AGREE technique for the RP-HPLC method was 0.74, while it was 0.81 for the TLC-densitometric method as presented in (Fig. 5).
Green analytical procedure index
The GAPI [25] is a reliable system used for assessment of the whole analytical procedure including parameters from sample preparation to the end of analysis. The GAPI system is separated into five major aspects which explains different parameters in each section. First one is the collection, preservation, transport, and storage of samples. The second category is sample preparation. The third one is solvents and reagents. The fourth is instrumentation and finally the fifth is overall method's type.
The GAPI technique is presented as three-colored pictogram according to the safety of the process in each section (Fig. 6) where RP-HPLC method resulted in four green, seven yellow and four red parts. On the other hand, TLC-densitometric method resulted in four green, eight yellow, and three red parts.
National environmental method index
The NEMI is another tool for evaluation of the greenness of the developed RP-HPLC and TLC-densitometric methods according to four requirements: waste, corrosiveness, poisonous chemical deposition, and dangerous chemical intake [26].
In the developed RP-HPLC and TLC-densitometric methods, methanol, water and ethyl acetate were used which were not regarded as PBT or hazardous solvents according to the EPA’s Toxic Release Inventory [26]. The pH of the mobile phase ranged from 2 to 12 is not considered as corrosive one (< 12) and the waste production from each run was 10.4 mL/sample in RP-HPLC method while it was 1.5 mL/sample in TLC-densitometric method.
Those results indicate that the developed methods meet the four requirements of greenness profile hence can be considered as ecofriendly methods as shown in (Fig. 7).
Conclusion
Green, simple, sensitive, and validated chromatographic methods were developed for the first time for simultaneous estimation of GEM and SOR used for treatment of NSCLC. RP-HPLC method has different advantages of being highly sensitive, rapid, and reproducible for separation and analysis of compounds in their mixture. On the other hand, TLC-densitometric method is a preferred method for routine analysis due to minimal amounts of solvents coupled with simpler and shorter analysis times. Additionally, using green solvents and excluding the use of harmful solvents is one of the most advantages of the developed methods to keep safety of environment and human health.
Moreover, pharmacokinetic study was achieved using those methods for both drugs after intra-peritoneal administration to rats either single or in combination. The pharmacokinetic parameters revealed that the coadministration of GEM and SOR significantly decreased the clearance (Cl) and volume of distribution (Vd) and increased significantly t half (t 1/2) and area under the plasma concentration–time curve (AUC0–t) of GEM. Also, its combination decreased significantly the clearance (Cl) and volume of distribution (Vd) and increased significantly area under the plasma concentration–time curve (AUC0–t) of SOR. These results showed that drug–drug interaction was occurred in rats. The obtained results forwarded the importance for further clinical studies and drug monitoring when administered GEM and SOR together.
Availability of data and materials
Available when request.
Abbreviations
- LC:
-
Lung cancer
- NSCLC:
-
Non-small cell lung cancer
- GEM:
-
Gemcitabine
- SOR:
-
Sorafenib
- SIL:
-
Sildenafil
- IS:
-
Internal standard
- RSD:
-
Relative standard deviation
- CV:
-
Coefficient of variation
- LLOQ:
-
Lower limit of quantification
- ULOQ:
-
Upper limit of quantification
- QCs:
-
Quality control samples
- LQC:
-
Low quality control
- MQC:
-
Middle quality control
- HQC:
-
High quality control
- K:
-
Elimination rate constant
- Vd:
-
Volume of distribution
- Cl:
-
Clearance
- AUC0–t:
-
Area under the plasma concentration–time curve
- THU:
-
Tetrahydrouridine
- 2' dFdU:
-
2' Difluorodeoxyuridine
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (6):394–424
Siegel R, Ma J, Zou Z, Jemal A (2013) Cancer statistics, 2013. CA: a cancer journal for clinicians 63:11–30
Das M, Jain R, Agrawal AK, Thanki K, Jain S (2014) Macromolecular bipill of gemcitabine and methotrexate facilitates tumor-specific dual drug therapy with higher benefit-to-risk ratio. Bioconjug Chem 25(3):501–509
Kohutek F, Stratena M, Rosik A, Tamasova M, Bystricky B (2016) First-line treatment of nonsquamous NSCLC using gemcitabine: a retrospective study of real-life practice. Lung cancer management 5(3):123–130
Takezawa K, Okamoto I, Yonesaka K, Hatashita E, Yamada Y, Fukuoka M, Nakagawa K (2009) Sorafenib inhibits non–small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Can Res 69(16):6515–6521
Jean GW, Mani RM, Jaffry A, Khan SA (2016) Toxic effects of sorafenib in patients with differentiated thyroid carcinoma compared with other cancers. JAMA Oncol 2(4):529–534
Pasqualetti G, Ricciardi S, Mey V, Del Tacca M, Danesi R (2011) Synergistic cytotoxicity, inhibition of signal transduction pathways and pharmacogenetics of sorafenib and gemcitabine in human NSCLC cell lines. Lung Cancer 74(2):197–205
Jiang S, Wang R, Zhang X, Wu F, Li S, Yuan Y (2020) Combination treatment of gemcitabine and sorafenib exerts a synergistic inhibitory effect on non-small cell lung cancer in vitro and in vivo via the epithelial-to-mesenchymal transition process. Oncol Lett 20(1):346–356
Yılmaz B, Kadıoğlu YY (2004) Comparison of zero-and second-order derivative spectrophotometric and HPLC methods for the determination of gemcitabine in human plasma. Il Farmaco 59(5):425–429
Freeman KB, Anliker S, Hamilton M, Osborne D, Dhahir PH, Nelson R, Allerheiligen SR (1995) Validated assays for the determination of gemcitabine in human plasma and urine using high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl 665(1):171–181
Kirstein MN, Hassan I, Guire DE, Weller DR, Dagit JW, Fisher JE, Remmel RP (2006) High-performance liquid chromatographic method for the determination of gemcitabine and 2′, 2′-difluorodeoxyuridine in plasma and tissue culture media. J Chromatogr B 835(1–2):136–142
Lanz C, Früh M, Thormann W, Cerny T, Lauterburg BH (2007) Rapid determination of gemcitabine in plasma and serum using reversed-phase HPLC. J Sep Sci 30(12):1811–1820
Yılmaz B, Kadıoğlu YY, Aksoy Y (2003) Simultaneous determination of gemcitabine and its metabolite in human plasma by high-performance liquid chromatography. J Chromatogr B 791(1–2):103–109
Bowen C, Wang S, Licea-Perez H (2009) Development of a sensitive and selective LC–MS/MS method for simultaneous determination of gemcitabine and 2, 2-difluoro-2-deoxyuridine in human plasma. J Chromatogr B 877(22):2123–2129
Khoury H, Deroussent A, Reddy LH, Couvreur P, Vassal G, Paci A (2007) Simultaneous determination of gemcitabine and gemcitabine-squalene by liquid chromatography–tandem mass spectrometry in human plasma. J Chromatogr B 858(1–2):71–78
Wang L-Z, Yong W-P, Soo R-A, Lee S-C, Soong R, Lee H-S, Goh B-C (2009) Rapid determination of gemcitabine and its metabolite in human plasma by LC-MS/MS through micro protein precipitation with minimum matrix effect. J Pharm Sci Res 1(3):23–32
Abdelhameed AS, Attwa MW, Attia MI, Alanazi AM, Alruqi OS, AlRabiah H (2022) Development of novel univariate and multivariate validated chemometric methods for the analysis of dasatinib, sorafenib, and vandetanib in pure form, dosage forms and biological fluids. Spectrochim Acta Part A Mol Biomol Spectrosc 264:120336
Sharma T, Khurana RK, Borges B, Kaur R, Katare O, Singh B (2021) An HPTLC densitometric method for simultaneous quantification of sorafenib tosylate and chrysin: analytical method development, validation and applications. Microchem J 162:105821
Blanchet B, Billemont B, Cramard J, Benichou A, Chhun S, Harcouet L, Ropert S, Dauphin A, Goldwasser F, Tod M (2009) Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J Pharm Biomed Anal 49(4):1109–1114
Khan I, Iqbal Z, Khan A, Hassan M, Nasir F, Raza A, Ahmad L, Khan A, Mughal MA (2016) A simple, rapid and sensitive RP-HPLC-UV method for the simultaneous determination of sorafenib & paclitaxel in plasma and pharmaceutical dosage forms: application to pharmacokinetic study. J Chromatogr B 1033:261–270
Zhao M, Rudek MA, He P, Hafner F-T, Radtke M, Wright JJ, Smith BD, Messersmith WA, Hidalgo M, Baker SD (2007) A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B 846(1–2):1–7
Jain L, Gardner ER, Venitz J, Dahut W, Figg WD (2008) Development of a rapid and sensitive LC–MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal 46(2):362–367
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical eco-scale for assessing the greenness of analytical procedures. TrAC Trends in Anal Chem 37(14):61–72
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—Analytical GREEnness metric approach and software. Anal Chem 92(14):10076–10082
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181(1):204–209
Elzanfaly ES, Hegazy MA, Saad SS, Salem MY, Abd El Fattah LE (2015) Validated green high-performance liquid chromatographic methods for the determination of coformulated pharmaceuticals: a comparison with reported conventional methods. J Sep Sci 38(5):757–763
Garber CJ, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J (2011) Guide For the Care and Use of Laboratory animals, 8thed, National Research Council 28. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Bioanalytical method validation guidance for industry, 2013
U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) (2013) Bioanalytical method validation guidance for industry
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Author information
Authors and Affiliations
Contributions
N.R. carried out the experiment. N.R. and M.M. wrote the manuscript. N.H. and M.M. prepared figures and tables. M.T. supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
All animal research was achieved according to the Ethics Committee for Animal Experimentation (Institutional Animal Care and Use Committee, Beni-Suef University) with approval number: 022–255.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharkawi, M.M.Z., Mohamed, N.R., El-Saadi, M.T. et al. FDA-Validated Ecofriendly Chromatographic Methods for Simultaneous Determination of Gemcitabine and Sorafenib: Applications to Pharmacokinetics. Chromatographia 86, 109–123 (2023). https://doi.org/10.1007/s10337-022-04232-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04232-1