Abstract
Capillary electrophoresis was chosen as cost-effective and robust method to separate ketamine enantiomers. For the method development, first different native and modified cyclodextrins were tested. The most promising chiral selector was α-cyclodextrin. A design of experiments (DoE) was carried out, which started with the screening of relevant factors. Based on these results, the method was optimized according to the significant factors (buffer, cyclodextrin concentration, pH value, voltage, temperature) of the screening based on the response resolution and migration time of the later migrating enantiomer. The optimized conditions consisted of a background electrolyte with 275 mM TRIS, adjusted with 85% phosphoric acid to a pH of 2.50, and 50 mM α-cyclodextrin, at a temperature of 15 °C, an applied voltage of 30 kV and an injection pressure of 1.0 psi for 10 s. A fused-silica capillary with a total length of 70 cm and an effective length to the detector of 60 cm was used. The method was validated according to ICH guideline Q2 R(1). The limit of quantification was 3.51 µg mL−1 for S-ketamine and 3.98 µg mL−1 for R-ketamine. The method showed good linearity for racemic ketamine with R2 of 0.9995 for S-ketamine and 0.9994 for R-ketamine. The lowest quantifiable content of S-ketamine found in R-ketamine was 0.45%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
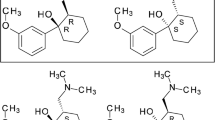
Ketamine has been used in drug therapy for over 50 years. Due to its antagonistic effect on the NMDA receptor, it is mainly applied in anesthesia [1]. Recently, research has also been conducted into other areas of application such as neuropathic pain and depression [2]. The dose-dependent effects are particularly interesting. Subanesthetic doses lead to psychotic states such as hallucinations, floating sensations, and a lack of sense of time and space [3]. The effect of ketamine is not only limited to the NMDA receptor but also acts on other receptors, which causes many physiological effects. Among them are muscarinic, nicotinic, and opioid receptors, thus ketamine acts as a so-called dirty drug [4]. Because of its psychotic’s effects, ketamine is abused as a party drug [5] and is therefore subject to strong legal restrictions on trade and therapy. Ketamine consists of two stereoisomers, which also differ in their effects. The S-enantiomer shows a higher potency than the R-enantiomer at the NDMA receptor and thus shows a higher anesthetic effect [6]. However, both the racemate and the enantiomerically pure S-ketamine are commercially available. To ensure the enantiomeric purity of S-ketamine, the European Pharmacopoeia makes use of HPLC with a chiral AGP column [7]. This column and chiral cellulose columns have already been used for the chiral separation of ketamine enantiomers by means of HPLC [8,9,10]. Unfortunately, chiral columns are very expensive. A cheaper alternative is chiral capillary electrophoresis (CE). With mainly cyclodextrins as chiral selectors [11], it is possible to easily separate the enantiomers from each other, as different diastereomeric complexes of the enantiomers are formed with the cyclodextrin. In the past, some methods have been published that use negatively charged sulphated cyclodextrins to separate the ketamine enantiomers [12,13,14,15,16,17,18]. However, as the degree of substitution of the cyclodextrin of different batches may differ [19], separations can be negatively affected, resulting in worse results. Single isomer cyclodextrins which do not have this disadvantage are to be preferred but are much more expensive due to the more complex production. Methods that have used native cyclodextrins such as α-cyclodextrin [20,21,22], β-cyclodextrin [23] and γ-cyclodextrin [24] have been published as well. The aim of this work was to develop a well reproducible and cheap method based on previous publications to improve the separation of the enantiomers of ketamine and to assess the enantiomeric purity of the respective enantiomer by means of CE. The focus was on achieving baseline separation with improved resolution using a native cyclodextrin and design of experiments (DoE) to statistically validate the method development.
Materials and Methods
Chemicals
Sodium hydroxide platelets, phosphoric acid 85%, tris-(hydroxymethyl)-aminomethane (TRIS), β-cyclodextrin sulphated sodium, carboxymethyl-β-cyclodextrin sodium salt (CM-β-CD), 2-hydroxypropyl-β-cyclodextrin, β-cyclodextrin, methyl-β-cyclodextrin, heptakis-(2,3,6-tri-O-methyl)-β-cyclodextrin (TM-β-CD), racemic ketamine hydrochloride and R-ketamine hydrochloride were purchased from Sigma Aldrich (Darmstadt, Germany). α-cyclodextrin and γ-cyclodextrin were obtained from Wacker Chemie (München, Germany). Heptakis-(2,3-di-O-acetyl-6-sulfo-)-β-cyclodextrin (HDAS-β-CD) was bought from Regis Technologies (Morton Grove, Texas, US), 0.1 M sodium hydroxide solution from VWR Chemicals (Darmstadt, Germany), hydrochloric acid 37% from Bernd Kraft (Duisburg, Germany) and neostigmine bromide from Roche (Grenzach, Germany). Deionized water was obtained from an in-house water purification system from Merck Millipore (Darmstadt, Germany).
Sample and Buffer Preparation
The background electrolyte (BGE) was prepared by weighing the appropriate amount of TRIS and adjusting it to the respective pH value with 85% phosphoric acid. The chiral selector was weighed in and dissolved in the BGE. For validation, stock solutions of 658.1 µg mL−1 racemic ketamine, 137.1 µg mL−1 R-ketamine and 274.2 µg mL−1 neostigmine bromide, as well as stock solutions of 1000 µg mL−1 R-ketamine, 187.5 µg mL−1 racemic ketamine and 750 µg mL−1 of neostigmine bromide in deionized water for the determination of the lowest amount of S-ketamine in R-ketamine. For the individual samples and the calibration points, the respective volumes of the stock solutions were transferred to a 5.0 mL flask and diluted with deionized water. For the determination of the lowest amount of S-ketamine the respective volumes of the stock solutions were mix up with deionized water to a total volume of 750 µL. Prior to use, BGE and sample solutions were filtered using a 0.45 µm PVDF filter (Carl Roth, Karlsruhe, Germany).
Instrumentation
pH measurements were performed with a pH meter from Metrohm (Filderstadt, Germany). The CE system used was a P/ACE MDQ system from Sciex (Darmstadt, Germany) with a photodiode array detector.
Electrophoretic Conditions
A fused silica capillary from BGB Analytik Vertrieb (Rheinfelden, Germany) with an internal diameter of 50 µm, a total length of 70 cm and an effective length of 60 cm was chosen for the separation. Samples were injected at a pressure of 1.0 psi for 10.0 s. The capillary was conditioned first with 1.0 M sodium hydroxide solution, second with 2.0 M hydrochloric acid and third with deionized water at a pressure of 30.0 psi, each for 10.0 min. Subsequently, the capillary was rinsed with BGE for 2 min and a voltage of 20.0 kV for 20 min was applied. Before each sample injection, the capillary was rinsed 1.0 min with 0.1 M sodium hydroxide, 1.0 min deionized water and 2.0 min with BGE at a pressure of 20.0 psi. Analytes were detected at a wavelength of 194 nm.
Software
Data evaluation of the CE measurements was performed with 32 Karat Software 8.0 from Sciex (Darmstadt, Germany). The design of the experimental plans and the statistical evaluation were carried out with design expert 12.0 (Minneapolis, Minnesota, US).
Results and Discussion
The aim was to develop a well reproducible and cheap method for the separation of ketamine enantiomers. Several methods are described in the literature, which used mainly a TRIS buffer or phosphate buffer with an acidic pH value and a negatively charged sulfated cyclodextrin as chiral selector [13,14,15,16,17,18]. Inhomogeneously substituted cyclodextrins can have a negative influence on separation, as already mentioned. To circumvent this problem and to avoid obtaining expensive single isomer cyclodextrins, special attention had to be paid to native cyclodextrins, where also already some methods were published [20,21,22,23,24]. Fillet et al. developed a procedure for the separation of enantiomers with neutral and charged cyclodextrins [25]. For basic drugs, like ketamine, a 100 mM phosphate buffer adjusted to pH 3.00 with triethanolamine is recommended. Starting concentrations of the chiral selectors are 5 mM for charged and 15 mM for neutral cyclodextrins. α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin, methyl-β-cyclodextrin, TM-β-CD as neutral cyclodextrins were tested and sulphated β-cyclodextrin, CM-β-CD and HDAS-β-CD as charged cyclodextrins, respectively. For all charged cyclodextrins, no separation occurred either at a positive voltage or negative voltage, even when the cyclodextrin concentration was increased up to 50 mM. α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin showed a separation but not yet a baseline separation, while TM-β-CD and methyl-β-CD showed no separation. Example electropherograms are shown in Fig. S1. When the concentration of the respective chiral selector was increased under positive voltage, the migration times increased as well but the resolution improved. The best results were found with α-cyclodextrin and γ-cyclodextrin. Further experiments were carried out with these two cyclodextrins. A TRIS phosphate buffer as well as a pure phosphate buffer with pH 3.0 were tried out as an alternative background electrolyte. Only when the temperature was lowered, both enantiomers could be baseline separated from each other. Similar observation was reported by Amini et al. [20]. The combination of TRIS buffer with α-cyclodextrin and low temperature showed the best results with an improved resolution compared to previously published methods.
Screening
Based on the results of the preliminary investigations, the pH value of the separation buffer, the concentration of the TRIS buffer and α-cyclodextrin, temperature and voltage were selected as factors with which screening and further method optimization should be carried out. A screening experiment should provide as much information as possible with as few experiments as possible. A face-centered central composite (FCCC) design with six center points was used as the experimental design for the screening. A two-stage central composite (CCC) design could not be performed because the CE system used could not cool the capillary below 15 °C, therefore there is a limitation to the factor level. The following parameters were chosen as the starting and center point: 200 mM TRIS buffer, adjusted to pH 3.0 with phosphoric acid, 50 mM α-cyclodextrin, 17.5 °C and a voltage of 27.5 kV. As an analytical response, the resolution based on Eq. 1 and the migration time of the later migrating enantiomer were chosen
The experimental plan with the respective factor levels and the obtained results can be found in Table 1. The running order was randomized. Regarding the buffer concentration, it could be seen, that an increase led to a prolongation of the migration time but also to a better resolution. A higher voltage led to significantly reduced migration times and at the same time also increased the resolution. Increasing the α-cyclodextrin concentration only increased the migration time and did not improve the resolution significantly. The selected factor level concentration of 60 mM already seems to be close to the optimum for chiral separation. The same effect can be seen with the change in the pH value, whereby a reduction in the pH value showed a minimal improvement in the resolution. Besides the buffer concentration, a change in temperature showed the greatest impact. Reducing the temperature produced better resolution, but also greatly slowed down migration times.
An analysis of variance (ANOVA) was performed on the experimental data. Insignificant factors were removed one after another to improve the quality of the model. If insignificant factors were involved in interactions which are significant, the factors were included in the model. The equations were recalculated after each factor elimination. Buffer concentration, temperature and voltage were identified as a significant factor with buffer concentration and temperature being the ones with the highest influence.
For the migration time, the statistical analysis is somewhat different. It is in the nature of the method used that all selected factors would have a significant influence. A higher buffer concentration slows down migration due to a higher ionic strength. A further slowdown is seen with an increased cyclodextrin concentration. A faster migration is obtained with a higher voltage and a higher temperature. An increase in pH value in the range of 2.5–8.0 is accompanied by a stronger EOF, which usually accelerates migration. Here, however, an increase showed a slight opposite effect. The greatest influence was shown by temperature and voltage.
The coefficient of variation R2 shows the fitting between experimental and modelled values. R2adj. on the other hand, refers to the number of parameters used in the model. With R2 = 0.9702 and R2adj. = 0.9404 for the resolution and R2 = 0.9738 and R2adj. = 0.9505, respectively, for the migration time the data and the model showed a good fit, and the models can be used.
Method Optimization
Buffer concentration, temperature and α-cyclodextrin concentration were selected as factors to be optimized. Since a high voltage resulted in both faster analysis times and better resolution, a maximum voltage of 30 kV was used for the method optimization. A lower pH value resulted in slightly better resolution. Therefore, a pH value of 2.5 was selected for the optimization. This has the additional side effect that the electroosmotic flow has little to no influence on the analysis. Since the used chiral selector is the most expensive component and the concentration of 60 mM in the screening experiments was already near the optimum, the concentration of the chiral selector was chosen as a factor to find out whether a lower concentration also led to a good analysis result besides the non-significantly influence on the separation to save costs. An FCCC design with six center points was selected for the method optimization. The following parameters were chosen as the starting and center point: 250 mM TRIS buffer, adjusted to pH 2.5 with phosphoric acid, 55 mM α-cyclodextrin, 16.0 °C. The voltage was held constant for all runs at 30 kV. The varied factor levels can be seen in Table 2.
An ANOVA was also performed for the method optimization. The modelling was analogous to the screening. To fit the model, the insignificant factors were excluded. The model for the resolution was based on a linear function and for the migration time on a quadratic function, respectively. The 3D surface plots of the factors and their response can be seen in Fig. 1.
With R2 = 0.9204 and R2adj. = 0.9111 for the resolution and R2 = 0.9991 and R2adj. = 0.9986, respectively, for the migration time the data and the model showed a good fit, and an optimization can be carried out. The software´s own optimization function was used. The highest resolution with the shortest migration time should be the goal, while the resolution should be at least 1.9. All conditions were of equal importance.
The most optimal condition was found to be a 275 mM TRIS/phosphate buffer with pH 2.5 and 50 mM α-cyclodextrin at a temperature of 15 °C while applying a constant voltage of 30 kV. An electropherogram with the optimized conditions is shown in Fig. 2.
Electropherogram of racemic ketamine with neostigmine bromide with optimized conditions: 275 mM TRIS buffer adjusted with 85% phosphoric acid to a pH value of 2.50, 50 mM α-cyclodextrin, 15 °C, 30 kV, injection at 1.0 psi for 10 s, fused silica capillary with a total length of 70 cm and an effective length of 60 cm, internal diameter of 50 µm, wavelength: 194 nm
Analytical Performance/Validation
The developed method has been validated according to the ICH Q2 (R1) guideline [26]. The parameters precision, accuracy, specificity, limit of quantification (LOQ), linearity, working range and robustness were evaluated. To determine the precision, three racemic solutions with concentrations of 16.5, 38.4 and 60.3 µg mL−1 were injected on every two different days (n = 3). An additional 35.1 µg mL−1 neostigmine bromide was added as an internal standard to minimize the influence of UV detectors fluctuations. The parameters to be tested were the migration time of both enantiomers and their resolution. The accuracy was checked by means of the recovery rate.
For this purpose, three solutions of 8.2 µg mL−1 racemic ketamine were prepared and spiked with 2.74, 13.7 and 21.9 µg mL−1 of R-ketamine to obtain three different concentration levels (n = 3) and an overlay of the electropherograms is shown in Fig. 3. These solutions were also injected on two different days and quantified. The exact results are listed in Table 3. The solution with an additional 21.9 µg mL−1 R-ketamine was used to determine the specificity of the method. The peak of R-ketamine is clearly larger than that of S-ketamine. An overlapping of the peaks is also not recognizable. Accordingly, the method is specific for the separation of both ketamine enantiomers and the migration order could be confirmed. For linearity, calibration curves with six different concentration levels of a racemic mixture were measured in triplicate in the range of 5.5–32.9 µg mL−1 per enantiomer. The coefficient of determination of 0.9995 for S-ketamine and 0.9994 for R-ketamine shows the good linearity of the method. The calibration curves and residual plots are shown in Fig. S2. With these calibration curves, the LOQ was determined according to Eq. 2 with s being the slope of the respective regression line and σ is the standard deviation of the response.
A LOQ of 3.51 µg mL−1 was determined for S-ketamine and a LOQ of 3.98 µg mL−1 for R-ketamine.
To determine the lowest possible content of S-ketamine in R-ketamine, linearity was determined in the range of 0.1–5.0% S-ketamine at a constant R-ketamine concentration of 500 µg mL−1. A coefficient of determination of 0.9996 also indicated good linearity. The calibration curve and residual plot are shown in Fig. S3. The LOQ of S-ketamine was determined again using Eq. 2 and was 2.24 µg mL−1 which corresponds to 0.45%. Figure 4 shows an example of an electropherogram of 0.50% S-ketamine in R-ketamine. Since the resolution according to Eq. 1 should only be used for peaks of equal height, the European pharmacopoeia determines the peak-to-valley ratio according to Eq. 3 for peaks with high concentration differences.
The peak-to-valley ratio of 0.50% S-ketamine in R-ketamine is 4.26 and thus there is still sufficient separation. The two LOQ determined are very similar with the slight difference being explained by the large excess of R-ketamine compared to the racemate. To check for accuracy, three samples were measured in triplicate on two different days with 0.75%, 1.5% and 3.0% S-ketamine. The recovery rate was in the range of 98.8% to 100.4%. The exact results are listed in Table S1.
A two-level fractional design was again performed to check robustness. pH-value, buffer and cyclodextrin concentration were selected as parameters. Resolution and migration time were also used as responses, as in screening and optimization. The factor levels and the results can be found in Table 4.
Of note, the statistical evaluation showed that the buffer concentration has a significant influence and is therefore not considered a robust parameter. This was foreseeable. However, the responses obtained are within an acceptable range. The worst resolution for racemic ketamine was found at 1.82, which still exceeds the pharmacopoeia criteria for baseline separation (> 1.5). The migration time increased by two minutes in the worst case. This means that even if a change in buffer concentration has a statistically significant effect on the result, they are still acceptable, and the method can therefore still be regarded as robust [27].
Conclusion
A CE method to separate both ketamine enantiomers with α-cyclodextrin as a neutral chiral selector was successfully developed. The resolution could be improved in comparison to existing methods using α-cyclodextrin as a chiral selector. The obtained resolution of 2.0 is in the range of methods with charged cyclodextrins (2.2 [13], 2.4 [14]), but the migration time is longer. However, the native cyclodextrin costs significantly less than charged cyclodextrins. Additionally, the separation is not negatively affected by the inhomogeneous substitution of charged cyclodextrins as long as non-single isomer cyclodextrins are used. To the best of our knowledge, the presented method is the only CE method for the separation of ketamine enantiomers that has been developed and validated by statistical experimental design. DoE was used for screening and method optimization as well as for the test of robustness. For the first two, an FCCC design was used, for robustness a two-level fractional design. The method was optimized for a minimum resolution of 1.9 of racemic ketamine and the fastest possible migration time of the later migrating enantiomer which was the R-ketamine. A separation with the optimized parameters was achieved within 30 min. The lowest possible quantifiable content of S-ketamine in R-ketamine was 0.45%
Upon validation, the method was found to be accurate, robust, specific, linear, and reproducible.
Data availability
Data is available from the authors upon request.
Abbreviations
- CE:
-
Capillary electrophoresis
- DoE:
-
Design of experiments
References
Kohtala S (2021) Ketamine—50 years in use: from anesthesia to rapid antidepressant effects and neurobiological mechanisms. Pharmacol Rep 73(2):323–345. https://doi.org/10.1007/s43440-021-00232-4
Das J (2020) Repurposing of drugs-the ketamine story. J Med Chem 63(22):13514–13525. https://doi.org/10.1021/acs.jmedchem.0c01193
Domino EF, Warner DS (2010) Taming the ketamine tiger. Anesthesiology 113(3):678–684. https://doi.org/10.1097/ALN.0b013e3181ed09a2
Sinner B, Graf B (2008) Ketamine 1:1
Liu Y, Lin D, Wu B, Zhou W (2016) Ketamine abuse potential and use disorder. Brain Res Bull 126:68–73. https://doi.org/10.1016/j.brainresbull.2016.05.016
White P, Ham J, Way W, Trevor A (1980) Pharmacology of ketamine isomers in surgical patients
EDQM (2022) Monograph Esketamine hydrochloride. In: European pharmacopeia online 10.8, Council of Europe, Straßbourg, France
Aboul-Enein HY, Islam MR (1992) Enantiomeric separation of ketamine hydrochloride in pharmaceutical formulation and human serum by chiral liquid chromatography. J Liq Chromatogr Relat Technol 15(18):3285–3293. https://doi.org/10.1080/10826079208020884
Geisslinger G, Menzel-Soglowek S, Kamp H-D, Brune K (1991) Stereoselective high-performance liquid chromatographic determination of the enantiomers of ketamine and norketamine in plasma. J Chromatogr B Biomed Appl 568(1):165–176. https://doi.org/10.1016/0378-4347(91)80350-L
Svensson J-O, Gustafsson LL (1996) Determination of ketamine and norketamine enantiomers in plasma by solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Appl 678(2):373–376. https://doi.org/10.1016/0378-4347(95)00545-5
Juvancz Z, Szejtli J (2002) The role of cyclodextrins in chiral selective chromatography. Trends Analyt Chem 21(5):379–388. https://doi.org/10.1016/S0165-9936(02)00506-X
Cherkaoui S, Veuthey J-L (2002) Use of negatively charged cyclodextrins for the simultaneous enantioseparation of selected anesthetic drugs by capillary electrophoresis–mass spectrometry. J Pharm Biomed Anal 27(3–4):615–626
Kwaterczak A, Duszczyk K, Bielejewska A (2009) Comparison of chiral separation of basic drugs in capillary electrophoresis and liquid chromatography using neutral and negatively charged cyclodextrins. Anal Chim Acta 645(1–2):98–104. https://doi.org/10.1016/j.aca.2009.04.049
Porpiglia N, Musile G, Bortolotti F, De Palo EF, Tagliaro F (2016) Chiral separation and determination of ketamine and norketamine in hair by capillary electrophoresis. Forensic Sci Int 266:304–310. https://doi.org/10.1016/j.forsciint.2016.06.017
Sandbaumhüter FA, Theurillat R, Thormann W (2017) Separation of hydroxynorketamine stereoisomers using capillary electrophoresis with sulfated β-cyclodextrin and highly sulfated γ-cyclodextrin. Electrophoresis 38(15):1878–1885. https://doi.org/10.1002/elps.201700016
Stalcup AM, Gahm KH (1996) Application of sulfated cyclodextrins to chiral separations by capillary zone electrophoresis. Anal Chem 68(8):1360–1368. https://doi.org/10.1021/ac950764a
Theurillat R, Larenza MP, Feige K, Bettschart-Wolfensberger R, Thormann W (2014) Development of a method for analysis of ketamine and norketamine enantiomers in equine brain and cerebrospinal fluid by capillary electrophoresis. Electrophoresis 35(19):2863–2869. https://doi.org/10.1016/0378-4347(95)00545-5
Ying Kwan H, Thormann W (2012) Electrophoretically mediated microanalysis for characterization of the enantioselective CYP 3 A 4 catalyzed N-demethylation of ketamine. Electrophoresis 33(22):3299–3305. https://doi.org/10.1002/elps.201200127
Lurie IS, Odeneal NG, McKibben TD, Casale JF (1998) Effects of various anionic chiral selectors on the capillary electrophoresis separation of chiral phenethylamines and achiral neutral impurities present in illicit methamphetamine. Electrophoresis 19(16–17):2918–2925. https://doi.org/10.1002/elps.1150191620
Amini A, Sörman UP, Lindgren BH, Westerlund D (1998) Enantioseparation of anaestethic drugs by capillary zone electrophoresis using cyclodextrin-containing background electrolytes. Electrophoresis 19(5):731–737. https://doi.org/10.1002/elps.1150190522
Bingcheng L, Yibing J, Yuying C, Epperlein U, Koppenhoefer B (1996) Separation of drug enantiomers by capillary electrophoresis: α-cyclodextrin as chiral solvating agent. Chromatographia 42(1):106–110. https://doi.org/10.1007/BF02271064
Chankvetadze B, Burjanadze N, Breitkreutz J, Bergander K, Bergenthal D, Kataeva O, Blaschke G (2002) Mechanistic study on the opposite migration order of the enantiomers of ketamine with α-and β-cyclodextrin in capillary electrophoresis. J Sep Sci 25(15–17):1155–1166. https://doi.org/10.1002/1615-9314(20021101)25:15/17%3C1155::AID-JSSC1155%3E3.0.CO;2-M
Koppenhoefer B, Epperlein U, Christian B, Lin B, Ji Y, Chen Y (1996) Separation of enatiomers of drugs by capillary electrophoresis III. β-cyclodextrin as chiral solvating agent. J Chromatogr A 735(1–2):333–343. https://doi.org/10.1016/0021-9673(95)01210-9
Koppenhoefer B, Epperlein U, Christian B, Yibing J, Yuying C, Bingcheng L (1995) Separation of enantiomers of drugs by capillary electrophoresis I. γ-cyclodextrin as chiral solvating agent. J Chromatogr A 717(1–2):181–190. https://doi.org/10.1016/0021-9673(95)00556-5
Fillet M, Hubert P, Crommen J (1998) Method development strategies for the enantioseparation of drugs by capillary electrophoresis using cyclodextrins as chiral additives. Electrophoresis 19(16–17):2834–2840. https://doi.org/10.1002/elps.1150191608
ICH (2005) Guideline Q2(R1), validation of analytical procedures: text and methodology international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use
Vander Heyden Y, Nijhuis A, Smeyers-Verbeke J, Vandeginste B, Massart D (2001) Guidance for robustness/ruggedness tests in method validation. J Pharm Biomed Anal 24(5–6):723–753. https://doi.org/10.1016/S0731-7085(00)00529-X
Acknowledgements
Thanks are due to Karlo Heider for practical support in the lab.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by SS. The first draft of the manuscript was written by SS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
None of the authors of this paper does have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmidt, S., Holzgrabe, U. Method Development, Optimization, and Validation of the Separation of Ketamine Enantiomers by Capillary Electrophoresis Using Design of Experiments. Chromatographia 86, 87–95 (2023). https://doi.org/10.1007/s10337-022-04229-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04229-w