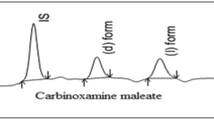
Abstract
Chiral discrimination of thyroxine (T4) enantiomers was performed using ultra high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) on a chiral crown ether-derived ChiroSil SCA (-) column. The different composition of mobile phases and the effect of column oven temperatures were investigated and the optimum chromatographic separation with respect to resolution and analysis time was achieved using a mixture of 60% methanol/water (v/v) with 0.1% formic acid at 40 °C having a flow rate of 1.4 mL min−1. The thermodynamic data from van’t Hoff plots of temperature experiments revealed that the enantioseparation was enthalpically favored process. The method was validated in the concentration range of 0.5–100 μg mL−1 for both enantiomers and proved to be rapid, precise, sensitive, and selective method for the enantiodiscrimination of T4 under the optimized conditions. The calibration curves of both D- and L-T4 showed an excellent linearity with coefficient of determination (R2) > 0.9997. The developed chiral method was successfully applied for a quantitative assay to check the enantiomeric purities of the six pharmaceutical formulations of levothyroxine sodium tablets and the enantiomeric impurities identified were in the range of 0.11–0.29%. This method could be applied for the determination of enantiomeric purity on pharmaceuticals and also for the monitoring of thyroid hormone levels.
Graphical Abstract






Similar content being viewed by others
References
Zhang Y, Shun Y, Hao Z, Hang S (2010) Chiral separation of pharmaceuticals by high performance liquid chromatography. Curr Pharm Anal 6:114–130
Nie Y, Liu X, Yang X, Zhao Z (2013) Recent application of chiral liquid chromatography-tandem mass spectrometric methods for enantiomeric pharmaceutical and biomedical determinations. J Chrom Sci 51:753–763
Adhikari S, Paik MJ, Lee W (2018) Liquid chromatographic enantiomeric separation of chiral aliphatic amines using 2-hydroxynaphthaldehyde as a derivatizing agent on polysaccharide-derived chiral stationary phases. Chromatographia 81:1337–1344
Yen PM (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142
Gika H, Lämmerhofer M, Papadoyannis I, Lindner W (2004) Direct separation and quantitative analysis of thyroxine and triiodothyronine enantiomers in pharmaceuticals by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 800:193–201
Jeon SH, Kim M, Han HK, Lee W (2010) Direct enantiomer separation of thyroxine in pharmaceuticals using crown ether type chiral stationary phase. Arch Pharm Res 33:1419–1423
Jansen HI, Bruinstroop E, Heijboer AC, Boelen A (2022) Biomarkers indicating tissue thyroid hormone status: ready to be implemented yet? J Endocrinol 253:21–45
Wang R, Jia ZP, Hu XL, Xu LT, Li YM, Chen LR (2003) Determination of serum thyroxine enantiomers in patients by liquid chromatography with a chiral mobile phase. J Chromatogr B 785:353–359
Jin D, Kumar AP, Song GC, Lee YI (2008) Determination of thyroxine enantiomers in pharmaceutical formulation by high-performance liquid chromatography-mass spectrometry with precolumn derivatization. Microchem J 88:62–66
Roberts CG, Ladenson PW (2004) Hypothyroidism. Lancet 363:793–803
Fallahi P, Ferrari SM, Ruffilli I, Ragusa F, Biricotti M, Materazzi G, Miccoli P, Antonelli A (2017) Advancements in the treatment of hypothyroidism with L-T4 liquid formulation or soft gel capsule: an update. Expert Opin Drug Deliv 14:647–655
Gorman CA, Jiang NS, Ellefson RD, Elveback LR (1979) Comparative effectiveness of dextrothyroxine and levothyroxine in correcting hypothyroidism and lowering blood lipid levels in hypothyroid patients. J Clin Endocrinol Metab 49:1–7
Abou-Basha LI, Aboul-Enein HY (1995) Enantiomeric separation and optical purity determination of thyroxine enantiomers in bulk and pharmaceutical formulations. Pharm Acta Helv 70:237–240
Ekborg-Ott KH, Kullman JP, Wang X, Gahm K, He L, Armstrong DW (1998) Evaluation of the macrocyclic antibiotic avoparcin as a new chiral selector for HPLC. Chirality 10:627–660
Gondova´ T, Petrovaj J, Sucha´ M, Armstrong DW (2011) Stereoselective HPLC determination of thyroxine enantiomers in pharmaceuticals. J Liq Chromatogr Relat Technol 34:2304–2314
Aboul-Enein HY, Ali I, Hyun MH, Cho YJ, Jin JS (2002) Effect of acidity on the enantiomeric resolution of thyroxine and tocainide by HPLC on a (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid column. J Biochem Biophys Methods 54:407–413
Hyun MH (2015) Development of HPLC chiral stationary phases based on (+)-(18-Crown-6)-2, 3, 11, 12-tetracarboxylic acid and their applications. Chirality 27:576–588
Adhikari S, Lee W (2018) Chiral separation using chiral crown ethers as chiral selectors in chirotechnology. J Pharm Inv 48:225–231
Bioanalytical method validation: guidance for industry, FDA-2013-D-1020–0039 (2018)
Wang D, Stapleton HM (2010) Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 397:1831–1839
Tanoue R, Kume I, Yamamoto Y, Takaguchi K, Nomiyama K, Tanabe S, Kunisue T (2018) Determination of free thyroid hormones in animal serum/plasma using ultrafiltration in combination with ultra-fast liquid chromatography-tandem mass spectrometry. J Chromatogr A 1539:30–40
Bowerbank SL, Carlin MG, Dean JR (2019) A direct comparison of liquid chromatography-mass spectrometry with clinical routine testing immunoassay methods for the detection and quantification of thyroid hormones in blood serum. Anal Bioanal Chem 411:2839–2853
Dossou KSS, Farcas E, Servais AC, Chiap P, Chankvetadze B, Crommen J, Fillet M (2012) Optimization of the liquid chromatography enantioseparation of chiral acidic compounds using cellulose tris (3-chloro-4-methylphenylcarbamate) as chiral selector and polar organic mobile phases. J Chromatogr A 1234:56–63
Panella C, Ferretti R, Casulli A, Cirilli R (2019) Temperature and eluent composition effects on enantiomer separation of carvedilol by high-performance liquid chromatography on immobilized amylose-based chiral stationary phases. J Pharm Anal 9:324–331
Asnin LD, Stepanova MV (2018) Van’t Hoff analysis in chiral chromatography. J Sep Sci 41:1319–1337
Chester TL, Coym JW (2003) Effect of phase ratio on van’t Hoff analysis in reversed phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy. J Chromatogr A 1003:101–111
Wani DV, Rane VP, Mokale SN (2018) Liquid chromatographic separation and thermodynamic investigation of lorcaserin hydrochloride enantiomers on immobilized amylose-based chiral stationary phase. Chirality 30:284–292
Acknowledgements
This research was supported by Health Fellowship Foundation, 2021, South Korea.
Funding
Health Fellowship Foundation, 2021
Author information
Authors and Affiliations
Contributions
Experiment, data collection and analysis were performed by J. Lee. Interpretation of the data and writing of the original manuscript was done by S. Adhikari. Conceptualization, experimental design, editing and supervision were contributed by W. Lee. Analysis tools, financial support, investigation and supervision were provided by H.-R.Yoon.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that there is no conflict of interest to report regarding the publication of this research article.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J., Adhikari, S., Lee, W. et al. Development of Ultra High-Performance Liquid Chromatography–Tandem Mass Spectrometry Method for Enantiomer Resolution of Thyroxine on a Chiral Crown Ether Derived Chiral Stationary Phase. Chromatographia 86, 13–20 (2023). https://doi.org/10.1007/s10337-022-04219-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04219-y