Abstract
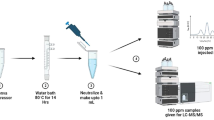
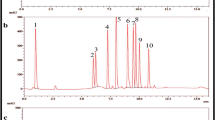
Pharmaceutical regulators are worried about medication quality and stability since drug degradation may result in harmful chemicals. Erlotinib (ERL) is a tyrosine kinase inhibitor associated with the epidermal growth factor receptor (EGFR) containing susceptible functional groups such as quinazoline and amine ketone, methoxy, and ethoxy leads to a reduction in pharmaceutical quality. According to the ICH-Q1A (R2) guideline, the goal of ERL stability studies is to establish its susceptibility to degradation under various environmental conditions. A novel isocratic stability–indicating liquid chromatography method has been developed using systemic quality by design (QbD) approach. The QbD strategy includes screening and optimization as phases. Placket Burman was used for primary parameters screening, and critical factors were optimized with response surface design. The prepared degradation samples (acid, base, neutral hydrolysis, oxidative, photolytic, and thermal) were separated using a Shimadzu GIST C18 column (250 mm × 4.6 mm, 5 µm) with 15 mM ammonium formate: ACN (58:42% v/v) as mobile phase, 0.9 mL/min flow rate, and 246 nm wavelength, which was found to be LC–MS compatible. A total of six degradation products (DPs) were identified with the optimized chromatography method. The drug was sensitive toward acidic and basic hydrolysis, but it remained stable under neutral, oxidative, thermal, and photolytic stress conditions. The optimized method was sensitive, specific, and robust, with linearity ranging from 10 to 35 µg/mL, with a correlation coefficient (R2 = 0.9997). The analytical method greenness score was calculated and observed that the developed method is green.







Similar content being viewed by others
Availability of Data
All the data generated or analyzed during this study are included in this published article and in form of supplementary data.
Abbreviations
- nm:
-
Nanometer
- ppm:
-
Parts per million
- °C:
-
Degree Celsius
- mL:
-
Milliliter
- µg:
-
Micron gram
- SIAM:
-
Stability indicating assay method
- mg:
-
Milligram
- Conc.:
-
Concentration
- HPLC:
-
High performance liquid chromatography
- LC/MS–MS:
-
Liquid Chromatography Mass Spectrometry
- HCl:
-
Hydrochloric acid
- NaOH:
-
Sodium hydroxide
- MeOH:
-
Methanol
- ACN:
-
Acetonitrile
- pH:
-
Potential of hydrogen
- Hrs:
-
Hours
- min:
-
Minute
- DOE:
-
Design of experiment
- QbD:
-
Quality by design
- ERL:
-
Erlotinib
- H2O2 :
-
Hydrogen peroxide
- PBD:
-
Plackett Burman design
- CCD:
-
Central composite design
- RSD:
-
Response surface design
- ANOVA:
-
Analysis of variance
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- ICH:
-
International Council for Harmonization
- DPs:
-
Degradation products
- CQAs:
-
Critical quality attributes
- CAAs:
-
Critical analytical attributes
- AMGS:
-
Analytical method greenness score
- CMAs:
-
Critical material attributes
References
Blessy M, Patel RD, Prajapati PN, Agrawal YK (2014) Development of forced degradation and stability indicating studies of drugs—a review. J Pharm Anal 4:159–165. https://doi.org/10.1016/J.Jpha.2013.09.003
Bajaj S, Singla D, Sakhuja N (2012) Stability testing of pharmaceutical products. J Appl Pharm Sci 2:129–138. https://doi.org/10.7324/JAPS.2012.2322
Baber N (1994) International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). Br J Clin Pharmacol 37(5):401. https://doi.org/10.1002/9781118445112.stat07000
Bakshi M, Singh S (2002) Development of validated stability-indicating assay methods—critical review. J Pharm Biomed Anal 28:1011–1040. https://doi.org/10.1016/S0731-7085(02)00047-X
Hirsh V, Thongprasert S, Campos D et al (2005) Erlotinib in previously treated non-small cell lung cancer. New Eng J. https://doi.org/10.1056/NEJMoa050753
Bareschino MA, Schettino C, Troiani T, Al Et (2007) Erlotinib in cancer treatment. Ann Oncol 18:35–41. https://doi.org/10.1093/Annonc/Mdm222
Field JK, Smith RA, Aberle DR, Al Et (2012) International association for the study of lung cancer computed tomography screening Workshop 2011 Report. J Thorac Oncol 7:10–19. https://doi.org/10.1097/JTO.0b013e31823c58ab
Fry WA, Menck HR, Winchester DP (1996) The national cancer data base report on lung cancer. Cancer 77:1947–1955. https://doi.org/10.1002/(SICI)1097-0142(19960501)77
Tóth G, Jánoska Á, Szabó ZI, Al Et (2016) Physicochemical characterisation and cyclodextrin complexation of erlotinib. Supramol Chem 28:656–664. https://doi.org/10.1080/10610278.2015.1117083
Diven DG, Bartenstein DW, Carroll DR (2015) Extending shelf life just makes sense. Mayo Clin Proc 90:1471–1474. https://doi.org/10.1016/J.Mayocp.2015.08.007
International Committee Harmonization (2018) International council for harmonisation of technical requirements for pharmaceuticals for human use final concept paper ICH Q14: analytical procedure development and revision of Q2(R1) analytical validation. https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf
Psimadas D, Georgoulias P, Valotassiou V, Loudos G (2012) Molecular nanomedicine towards cancer. J Pharm Sci 101:2271–2280. https://doi.org/10.1002/Jps.23146
Monks K, Molnár I, Rieger HJ, Al Et (2012) Quality by design: multidimensional exploration of the design space in high performance liquid chromatography method development for better robustness before validation. J Chromatogr A 1232:218–230. https://doi.org/10.1016/J.Chroma.2011.12.041
Kumar PA, Kumar YR, Jayashree A (2016) Development and validation of a stabilityindicating RP-HPLC method for the estimation of erlotinib impurities by Qbd approach. Rasayan J Chem 9:180–188. https://doi.org/10.1009/76-0083rjc.2016
Orlandini S, Pinzauti S, Furlanetto S (2013) Application Of quality by design to the development of analytical separation methods. Anal Bioanal Chem 405:443–450. https://doi.org/10.1007/S00216-012-6302-2
Kim MK, Park SC, Park G et al (2021) Analytical quality by design methodology for botanical raw material analysis: a case study of flavonoids In Genkwa Flos. Sci Reports 111(11):1–14. https://doi.org/10.1038/S41598-021-91341-W
Hicks MB, Farrell W, Aurigemma C et al (2019) Making the move towards modernized greener separations: introduction of the analytical method Greenness Score (AMGS) calculator. Green Chem 21:1816–1826. https://doi.org/10.1039/C8gc03875a
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—analytical greenness metric approach and software. Anal Chem 92:10076–10082. https://doi.org/10.1021/Acs.Analchem.0c01887
Saroj S, Shah P, Jairaj V, Rathod R (2018) Green analytical chemistry and quality by design: a combined approach towards robust and sustainable modern analysis. Curr Anal Chem 14:367–381. https://doi.org/10.2174/1573411013666170615140836
Gamal M, Naguib IA, Panda DS, Abdallah FF (2021) Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine: N-Butyl Bromide. Anal Methods 13:369–380. https://doi.org/10.1039/D0ay02169e
Naz A, Tabish I, Naseer A et al (2021) Green chemistry approach: method development and validation for identification and quantification of entecavir using FT-IR in bulk and pharmaceutical dosage form. Futur J Pharm Sci 71(7):1–8. https://doi.org/10.1186/S43094-021-00211-9
Latha ST, AnandaThangadurai S, Jambulingam M et al (2017) Development and validation Of RP-HPLC method for the estimation of erlotinib in pharmaceutical formulation. Arab J Chem 10:S1138–S1144. https://doi.org/10.1016/J.Arabjc.2013.02.006
Mahajan AA, Miniyar PB, Patil AS et al (2015) Separation, identification, and characterization of degradation products of erlotinib hydrochloride under ICH-recommended stress conditions by LC, LC-MS/TOF. J Liq Chromatogr Relat Technol 38:629–639. https://doi.org/10.1080/10826076.2014.936610
Babu C, Narasimha Rao KL, Devanna N, Suresh Reddy KVN (2016) Development and validation of a stability indicating HPLC method for the quantification of impurities in erlotinib hydrochloride dosage forms. Int J Res Pharm Sci 7:98–105. https://doi.org/10.10863/sc/583.2016
Pujeri SS, Khader AMA, Seetharamappa J (2009) Validated stability-indicating chromatographic method for the assay of erlotinib active pharmaceutical ingredient. Anal Lett 42:1855–1867. https://doi.org/10.1080/00032710903061170
Government of India, Ministry of Health (2018) The Indian Pharmacopeia Volume-II, Indian Pharmacopeia Commission Ghaziabad, 1969
Sharma G, Thakur K, Raza K, Katare OP (2019) Stability kinetics of fusidic acid: development and validation of stability indicating analytical method by employing analytical quality by design approach in medicinal product(S). J Chromatogr B 1120:113–124. https://doi.org/10.1016/J.JCHROMB.2019.05.001
Tome T, Žigart N, Časar Z, Obreza A (2019) Development and optimization of liquid chromatography analytical methods by using aqbd principles: overview and recent advances. Org Process Res Dev 23:1784–1802. https://doi.org/10.1021/ACS.OPRD.9B00238
Sahu PK, Ramisetti NR, Cecchi T, Al Et (2018) An overview of experimental designs In HPLC method development and validation. J Pharm Biomed Anal 147:590–611. https://doi.org/10.1016/J.JPBA.2017.05.006
Yao H, Vancoillie J, D’Hondt M, Al Et (2016) An analytical quality by design (Aqbd) approach for A L-asparaginase activity method. J Pharm Biomed Anal 117:232–239. https://doi.org/10.1016/J.JPBA.2015.08.042
Teng J, Zhu C, Lyu J et al (2022) Analytical lifecycle management (ALM) and analytical quality by design (Aqbd) for analytical procedure development of related substances in tenofovir alafenamide fumarate tablets. J Pharm Biomed Anal. https://doi.org/10.1016/J.JPBA.2021.114417
International Committee Harmonization (2015) International council for harmonization of technical requirements for pharmaceuticals for human use, ICH guideline Q9 on quality risk management. https://www.ich.org/page/quality-guidelines
Suryawanshi D, Jha DK, Shinde U, Amin PD (2019) Development and validation of a stability-indicating RP-HPLC method of cholecalciferol in bulk and pharmaceutical formulations: analytical quality by design approach. J Appl Pharm Sci 9:21–32. https://doi.org/10.7324/JAPS.2019.90604
Shinde DD, Ahirrao S, Prsad R (2018) Fishbone diagram: application to identify the root causes of student–staff problems in technical education. Wirel Pers Commun 1002(100):653–664. https://doi.org/10.1007/S11277-018-5344-Y
Patel MN, Kothari CS (2021) A comprehensive stability study of vardenafil using quality by design approach. Chromatogr 848(84):751–767. https://doi.org/10.1007/S10337-021-04059-2
Kurmi M, Kumar S, Singh B, Singh S (2014) Implementation of design of experiments for optimization of forced degradation conditions and development of a stability-indicating method for furosemide. J Pharm Biomed Anal 96:135–143. https://doi.org/10.1016/J.Jpba.2014.03.035
Youn BD, Choi KK (2004) A new response surface methodology for reliability-based design optimization. Comput Struct 82:241–256. https://doi.org/10.1016/J.Compstruc.2003.09.002
Snyder LR, Kirkland JJ, Glajch JL (2012) Basics of separation. Pract HPLC Method Dev. https://doi.org/10.1002/9781118592014.CH2
Vanaja K, Rani RHS (2007) Design of experiments: concept and applications of Plackett Burman design. Clin Res Regul Aff 24:1–23. https://doi.org/10.1080/10601330701220520
Lima EC, Gomes AA, Tran HN (2020) Comparison of the nonlinear and linear forms of the Van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° And ∆H°). J Mol Liq 311:113315. https://doi.org/10.1016/J.MOLLIQ.2020.113315
Gamal M, Naguib IA, Panda DS, Abdallah FF (2021) Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal Methods 13:369–380. https://doi.org/10.1039/D0AY02169E
Kelani KM, Elzanfaly ES, Saad AS, Al Et (2021) Different greenness assessment perspectives for stability-indicating RP-HPLC method used for the assay of isoxsuprine hydrochloride and four nephrotoxic and hepatotoxic photothermal degradation products. Microchem J 171:106826. https://doi.org/10.1016/J.MICROC.2021.106826
Funding
The authors have not received any fund for the work.
Author information
Authors and Affiliations
Contributions
All authors compiled, read and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gundecha, S., Patel, M. & Mayur, Y.C. An Application of Quality by Design and Analytical Greenness Assessment Approach for the Development of Erlotinib Stability Indicating Method. Chromatographia 85, 575–588 (2022). https://doi.org/10.1007/s10337-022-04167-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04167-7