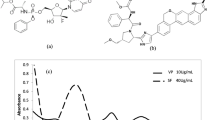
Abstract
Background
Chemical hazard is one of the most prominent side effects that come out along with the benefits of pharmaceutical production. Chemicals usage and waste production are involved in each step of production and are found to be in high percentage at the stage of quality testing. Conventional quality testing (assay) involves the use of solvents and reagents that generates high flammable and non-flammable waste and also enhanced the per batch cost of the medicine, whereas green chemistry offers a benign environment for drug designing, manufacturing, and analysis.
Results
In the present study, a green FTIR method for assay of antiviral drugs entecavir is developed and validated as per ICH Q2_R1 guidelines. A calibration curve is plotted between absorbance and concentration, yielding excellent linearity with a correlation coefficient (r2) value of 0.9991 in the concentration range of 0.25–0.75 mg.
Conclusion
The developed method was validated and was very specific, accurate (99.9–100%) at three levels of 80, 100, and 120% of test concentration and precise with < 1% RSD. The LOD of the method is 0.0674 mg and is able to quantify the active at the limit of 0.2042 mg. Four different drug brands available in the local market are assayed by the validated method, and %recoveries are found to be in the range of 99–101%.
Graphical abstract
Green Chemistry Approach: Method Development and Validation for Identification and Quantification of Entecavir using FT-IR in Bulk and Pharmaceutical Dosage Form

Similar content being viewed by others
Background
Entecavir, a selective HBV DNA polymerase inhibitor, is white or off-white crystal-like powdered available in ≥ 95% pure form and is slightly soluble in water but freely soluble in organic solvents (Fig. 1). On March 30, 2005, FDA approved entecavir for hepatitis B treatment [1]. Oral entecavir is prescribed as the first drug of choice in the treatment of hepatitis B. Entecavir is a selective HBV DNA polymerase inhibitor and, after oral administration, gets phosphorylated and converted into triphosphate, which is the active form (https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf) [2]. Entecavir is commercially available in various pharmaceutical dosage forms as a single medicament or combination with other therapeutic agents.
The FTIR spectrum of entecavir shows a prominent peak at wave number 3444 cm−1 because of the presence of amine (NH), at 1628 cm−1 carboxyl (RCOO-) group shows a sharp peak, at 1593cm−1 amide (NC) peak is present, at 1016 cm−1 carbonyl group (CO) exhibited a sharp peak, and tertiary amine (-CN) shows a strong signal at 1115 cm−1 [3].
Several analytical techniques have been reported for the identification, quantification, and impurity detection of entecavir in bulk, in plasma, peripheral blood, and dosage forms of different type, including the detection method used in these methods which includes liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) [4], fluorescence spectroscopy, colorimetric detection using UV–vis spectrometer and naked eye [5], LC-MS/MS [6], UPLC–MS/MS [7, 8], RP-HPLC [9,10,11,12,13], salting-out homogeneous liquid-liquid extraction LC-MS [14], UHPLC–MS/MS [15], powder X-ray diffractometry, and Raman spectroscopy [16, 17]. This comprehensive literature research reveals that these methods use a variety of solvents, reagents, and columns like methanol, acetonitrile, ammonium acetate, ammonia, solid-silver nanoparticles, formic acid, ammonium hydrogen carbonate, potassium phosphate buffer, deionized water, and other miscellaneous reagents and solvents of analytical grade. All these methods are time-consuming and based on complex solvent system procedures, which are less economical. The chances of troubleshooting also increased with the complexity and even lead to the need for an efficient technical person. This successful attempt of a simple, accurate, and precise analytical procedure via FTIR [18, 19] was made to quantify drug content in pure and dosage form.
Method
Materials
The working standard of entecavir was gifted from Fazal Elahi (Pvt) Ltd, and other excipients (Additional file 1) (Lactose, cellulose microcrystalline (Avicel PH101), croscarmellose sodium, P.V.P.K-30, magnesium stearate, triethanolamine, IPA (isopropyl alcohol)) were used from Sigma Aldrich and Merck Pvt Ltd. The entecavir brands (Cavira 0.5 mg, Envir 0.5 mg, Bvir 0.5 mg, and Ecavir 0.5 mg) were purchased from the local market and distilled water used from a lab.
Instrumentation
Analytical balance (Mettler Toledo with a working range of (0.01 to 60 g used as 5 digits), FTIR (IR Tracer-100 by Shimadzu installed with Lab Solutions IR), Sonicator (Transonic Digital S, USA), pharmaceutical grade marble or agate mortar, and pastel and mechanical shaker.
Method development
Preparation of blank
For making different aliquots, a blank were prepared using lactose (3.7485 g), Avicel PH101 (0.85 g), croscarmellose sodium (0.25 g), P.V.P.K-30 (0.10 g), and magnesium stearate (0.025 g).
Preparation of calibration concentrations or optimization and selection of wavelength
Nine calibration concentrations were prepared in a range of 0.25 to 0.75 mg of entecavir mixed with blank in different ratio to get final weight of 1 g (0.25+ 999.75, 0.30+999.70, 0.35+999.65, 0.40+999.60, 0.45+ 999.55, 0.55+ 999.45, 0.60+ 999.40, 0.65+999.35, 0.75 +999.25 mg).
Scanning the sample
The system was initialized, and the spectrum was converted into absorbance mode before taking the background scan. Background scan was taken with no sample in the sample compartment. After that, a small amount of calibration concentration sample was loaded into the sample holder by the ATR technique, and a scan was measured. In the same way, the remaining eight calibration concentration samples were scanned in absorbance mode.
Construction of the calibration curve
A calibration curve is created using the application “Quantitation” in LS-IR. The calibration curve requires the calibration parameter to be set as the Beer-Lambert’s law which dictates that in linear relationship among absorbance and concentration, the first order of equation was selected. The type of equation was set as peak intensity, and the peak of tertiary amine (–CN) at 1115 cm−1 for ECV was selected. The calibration curve was created using the relationship between the amount of the active present and absorbance of light by the particles of the active.
Preparation and scanning of unknown sample
An amount of 0.5 mg of entecavir was measured using a calibrated balance and transferred into a mortor. Then 999.5 mg of the blank was weighed and transferred into the same mortor. The powders were mixed properly. This sample was scanned in absorbance mode and saved.
Validation parameters performance
Validation of the developed method was performed as per ICH guidelines using the following parameters,
Specificity
The method specificity was determined by reading the scan of active alone and in overlay format with excipients in a detailed zoomed view, and the wavenumber was selected where there is no interference of other peaks.
Linearity and range
Nine concentrations of entecavir in the range of 0.25 to 0.75 mg were prepared, and the absorbance was determined. Linearity evaluated by linear regression analysis. The process was repeated three times, and the mean of all values (slope of the regression line, correlation coefficient, y-intercept, and residual sum of squares) were calculated.
Accuracy/recovery
Three concentrations of entecavir having 80, 100, and 120% levels were prepared in triplicate and scanned and quantified as unknown samples. The acceptable limit of active response ranges from 98 to 102%.
Precision
Method precision was assessed in two requisites which are repeatability (intra-day precision) and intermediate precision (inter-day precision) in compliance with ICH guidelines. Repeatability was performed by taking six determinations at 100% of the test concentrations or label claim of entecavir (0.5 mg) under the same experimental condition by the same analyst on the same day. Inter-day precision or intermediate precision was performed by repeating the process of repeatability by different analysts under the same experimental condition but on different days.
Limit of detection and quantitation
The LOD and LOQ were calculated by formula directed by ICH guideline by calculating curve slope and intercept standard deviation as mentioned below.
where the slope of the curve is denoted by S and σ represents the intercept standard deviation.
Ruggedness
Ruggedness refers to the degree of reproducibility of the drug. It was estimated by analyzing the same sample by two different analysts. The six determinations of 0.5 mg of entecavir were scanned under the same experimental conditions.
Application of developed method on the finished product
Twenty tablets of each of the four different brands named Bvir (0.5 mg), Cavira (0.5 mg), Envir (0.5 mg), and Ecavir (0.5 mg) were taken and crushed into powder using mortar and pestle. Accurately weight the powder equivalent to the weight of each tablet and scanned. The calibration curve ECV-1.istd. was loaded in the quantitation window, and all saved spectra of the finished product were imported over the calibration curve. The amount of entecavir was mention next to the absorbance value.
Result
Optimization of analytical procedures
In the present study, the selection of specific peak of entecavir (bulk and dosage form) was carried out by taking the IR spectrum of the pure form of active ingredient of entecavir (Fig. 2) absorbance mode and identified the prominent peaks which can easily be used for identification and quantification purposes. The spectrum shows various prominent peak which can be easily used, but among them, the selection of that specific peak, which does not get affected by any of its ingredient present in its dosage; scans of excipient were also recorded and thoroughly studied in overlay format. Entecavir gives a sharp peak of tertiary amine (-CN) at position 1115 cm−1; amine is not present in any of the excipient used to formulate the entecavir tablet dosage form; hence, the chances of interference of the peak in this region are minimal. Therefore, amine peak is selected for developing and validating an FTIR method for identification and quantification of entecavir.
IR-absorbance calibration curve for quantification of entecavir was constructed using 9 known concentrations. In selecting the intensity of the peak of the tertiary amine (-CN) in the first-order equation, against each concentration, their respective absorbance is calculated by the system. The calibration concentration was automatically constructed by LS-IR software, giving a value of correlation coefficient (r2) value of 0.9991. A curve was constructed between peak absorbance and concentration by keeping concentration as an independent variable on X-axis and peak absorbance as an independent variable on Y-axis. The result reveals that the relation between the drug concentration and absorbance complies with Beer-Lambert’s law in the range studied (0.25–0.75 mg), and the r2 value shows excellent linearity of relationship. The overlay scan of all nine concentrations and the calibration curve is mentioned in Figs. 3 and 4.
Validation of the developed method
The proposed assay method of entecavir for bulk and pharmaceutical dosage form has been validated in accordance with the guidelines given by the “International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use” (ICH). Entecavir was measured specifically without the inference of formulation ingredients. The developed method was found to be linear at these drug concentrations ranging from 0.25 to 0.75 mg (as per ICH guidelines, 80 and 120% of the test concentration should be covered in the calibration concentrations range, and that is included). The coefficient of regression (r2) value was computed to be Y = 0.03901X + 0.02158, presenting an r2 value of 0.9991, and the slope of the plot was 0.03901. The method was accurate with the mean recovery values of 99.95, 99.99, and 99.99% at 80, 100, and 120%, respectively. Furthermore, the %RSD was also found to be less than 1 for both intra and inter-day precision. The details of the accuracy and precision results are given in Tables 1 and 2. The limit of detection (LOD) of entecavir was found to be 0.0674 mg, while the limit of quantification (LOQ) was found as 0.2042 mg. The %RSD of the raggedness was found to be 0.0983.
Application of designed method on finished product
The developed and validated method was then applied to the marketed product. The %recovery against the claimed amount was calculated with the accepted criteria of ± 02% (98–102%) of the label claim. The results were 99.7, 100.10, 100, and 99.5%.
Discussion
The risks of chemical hazards remain constant throughout the life span of pharmaceutical manufacturing, right from the dispensing until the disposal of waste. Entecavir tablets have been prescribed worldwide for the first-line treatment of hepatitis B. FT-IR assay method is found to be the simplest, cheap, easiest, coast effective, and above all, green among all other analytical procedures. Specificity is the ability to estimate the analyte in the presence of components that may expect to be present, including formulation ingredients and impurities as well (https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf). An overlay spectrum of active and ingredient showed the absence of interference between the spectrum of standard drug, sample drug, and the placebo since none of the peaks appeared at wavenumber 1115 cm−1 except for amine.
The linearity of an analytical method is the ability to obtain test results (within a given range) directly proportional to the concentration (amount) of analyte in the sample. In the present study, linearity of the assay method was observed using 9 concentrations including 80, 100, and 120% of the test concentration. Figure 4 shows the plot between drug concentration and their corresponding absorption as per Beer-Lambert law. The correlation coefficient (r2) was found to be 0.9991, indicating excellent linearity (> 0.999).
Accuracy is the best indication of systematic errors. To ensure the accuracy of the newly developed method, three samples having three different drug concentrations were assessed. The mean percent recovery at various working strengths was > 99.90. The detailed observations of various drug concentration solutions are given in Table 1.
Precision was evaluated by repetitive intra-day determination of drug solution at two different time intervals. At the same time, inter-day precision was determined by conducting the same procedure by two analysts on two different days. The observations of intra and inter-day precisions are shown in Table 2.
The detection limit indicates the lowest amount of analyte in a sample that could be detected but not necessarily precisely quantified. The quantitation limit indicates the lowest amount of the analyte in a sample, which can be determined with suitable precision and accuracy and expresses the quantitation of parameters for quantitative assay for low levels of compounds in sample matrices and is also useful for the determination of impurities and/or degradation of products.
Conclusion
The developed FTIR method for identification and quantification of entecavir was validated in accordance with the ICH guideline, and this method appeared to be a suitable technique for the reliable analysis of commercial formulations containing entecavir. The most striking features of this method besides being green are its simplicity, specificity, linearity, accuracy, precision, and ruggedness. It is also an easier, rapid, and cost-effective method than HPLC and does not require the use of any expensive or toxic reagent. Hence, the present FTIR method is suitable for routine analysis of entecavir raw material and tablet dosage form.
Availability of data and materials
All the data are provided in the manuscript. Data and materials are available upon request.
Abbreviations
- FTIR:
-
Fourier transform infrared
- HPLC:
-
High-performance liquid chromatography
- RSD:
-
Relative standard deviation
References
Jung H (2018) Synthesis and physicochemical evaluation of entecavir-fatty acid conjugates in reducing food effect on intestinal absorption. Molecules 23:731. https://doi.org/10.3390/molecules23040731
Menendez AL, Alvarez M, Pacheco B (2014) Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr Opin Virol 8:1–9. https://doi.org/10.1016/j.coviro.2014.04.005
Rizwana BF (2018) Spectroscopic investigation, hirshfeld surface analysis and molecular docking studies on anti-viral drug entecavir. J Mol Structure 1164:447–458. https://doi.org/10.1016/j.molstruc.2018.03.090
Challa BR (2011) LC–ESI-MS/MS method for the quantification of entecavir in human plasma and its application to bioequivalence study. J Chromatogr B 879:769–776. https://doi.org/10.1016/j.jchromb.2011.02.023
Gao M (2014) Label-free silver nanoparticles for the naked eye detection of entecavir. Spectrochim Acta A Mol Biomol Spectrosc 126:178–183. https://doi.org/10.1016/j.saa.2014.02.009
Zhang D (2009) A sensitive method for the determination of entecavir at picogram per milliliter level in human plasma by solid phase extraction and high-pH LC–MS/MS. J Pharm Biomed Anal. 49:1027–1033. https://doi.org/10.1016/j.jpba.2009.02.003
De Nicolò A (2015) UPLC–MS/MS method for the simultaneous quantification of anti-HBV nucleos (t) ides analogs: entecavir, lamivudine, telbivudine and tenofovir in plasma of HBV infected patients. J Pharm Biomed Anal. 114:127–132. https://doi.org/10.1016/j.jpba.2015.05.016
Hou Z (2018) Simple and rapid UPLC-MS/MS method for quantification of entecavir in human plasma: Application to a bioequivalence study. Int J Clin Pharmacol Ther 56:197
Ashraf M, Hafiz MNS, Muhammad MH, Jameel R, Samina E, Muhammad I, Faiz-ul-Hassan N (2017) HPLC determination of entecavir in pure, tablet dosage form and spiked plasma. J Chem Soc Pakistan 39:1–5
Dalmora SL, Sangoi MDS, Nogueira DR, Silva LMD (2010) Validation of a stability-indicating RP-HPLC method for the determination of entecavir in tablet dosage form. J AOAC Int 93(2):523–530
Rambabu R, Subbarao J, Vidyadhara S (2014) Estimation and validation of Entecavir in bulk and pharmaceutical dosage forms by RP-HPLC. Int. J. Res. Ayurverda Pharm 5(4):531–535
Elzaher AA (2016) Validated spectrometric determination of penciclovir and entecavir in bulk and in pharmaceutical preparations. Bull Fac Pharm Cairo Univ 54:175–179
Satyanarayana L, Naidu SV, Rao MN, Priya LR, Suresh K (2011) The estimation of etecavir in tablet dosage form by RP-HPLC. Res J Pharm Technol 4(11):1699–1701
Zhao F-J (2012) Salting-out homogeneous liquid–liquid extraction approach applied in sample pre-processing for the quantitative determination of entecavir in human plasma by LC–MS. J Chromatogr B 881:119–125. https://doi.org/10.1016/j.jchromb.2011.12.003
Nicolò D (2016) UHPLC–MS/MS method with automated on-line solid phase extraction for the quantification of entecavir in peripheral blood mononuclear cells of HBV+ patients. J Pharm Biomed Anal 118:64–69
Kang Y (2018) Quantitation of polymorphic impurity in entecavir polymorphic mixtures using powder X-ray diffractometry and Raman spectroscopy. J Pharm Biomed Anal 158:28–37
Kang Y (2020) Low-content quantitation in entecavir tablets using 1064 nm Raman spectroscopy. J Spectrosc. https://doi.org/10.1155/2020/1308385
Nugrahani I (2019) FTIR-derivative as a green method for simultaneous content determination of caffeine, paracetamol, and acetosal in a tablet compared to HPLC. Vibrational Spectrosc 104:102941
Tiernan H (2020) ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochim Acta A Mol Biomol Spectrosc: 241:118636
Acknowledgements
The authors gratefully acknowledge the Dean of the Faculty of Pharmacy, University of Karachi, for his kind support.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
AN: Design, perform experiment, writing and supervise the project. IT: Performed all experiments. AN: Participated in experiment. AZS: Help in writingFAS: Help in writing. AZM: Help in writing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not Applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naz, A., Tabish, I., Naseer, A. et al. Green chemistry approach: method development and validation for identification and quantification of entecavir using FT-IR in bulk and pharmaceutical dosage form. Futur J Pharm Sci 7, 75 (2021). https://doi.org/10.1186/s43094-021-00211-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00211-9