Abstract
A method for the extraction and quantification of carvedilol, enalaprilat, and perindoprilat in 50 µL human plasma, using high-performance liquid chromatography with tandem mass spectrometry (LC–MS/MS) detection was developed and validated. Samples were prepared via protein precipitation with chromatographic separation on a Restek Ultra II Biphenyl column using gradient elution at a corresponding flowrate of 300 µL/min. Electrospray ionisation with mass detection at unit resolution in the multiple reaction monitoring (MRM) mode on an AB Sciex API 5500 mass spectrometer was used. Accuracy, precision, selectivity, sensitivity, matrix effects, recovery, process efficiency, and stability were assessed over the validation period. The assay was validated over the calibration range 0.2–200 ng/mL for all three analytes. The inter- and intra-day precision expressed as the coefficient of variation (CV) and accuracy (%Nom) all fell within acceptable limits. The overall recovery was calculated as 72.9%, 77.1%, and 77.0% for carvedilol, enalaprilat, and perindoprilat respectively, with the recovery being shown to be reproducible at the low, medium and high end of the calibration range for all three analytes. The method proved to be specific for all three analytes with no significant matrix effects observed. The validated method facilitated the analysis of carvedilol, enalaprilat, and perindoprilat in human plasma collected from adults as part of a pilot pharmacokinetic study. This validated analytical method lays the foundation for determining adherence in heart failure patients prescribed with carvedilol, enalapril and perindopril.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been estimated that there are approximately 26 million cases of Heart Failure (HF) worldwide [1]. Mortality rates for HF patients remain high with 17–45% of deaths occurring within 1 year of diagnosis [2], with Africa’s HF case fatality rates the highest in the world at 34% [3]. Poor treatment adherence to HF medicines results in poor health outcomes and increased health care costs [4, 5]. Readmissions and mortality have been reduced when interventions have been implemented aimed at increasing adherence [5, 6]. Medication adherence is therefore a critical component of HF self-care, with adherence rates varying from 10 to 98% depending on both the definition used to define adherence and the method used to analyse adherence [7]. Most authors indicate an average of 40–60% in terms of adherence to HF medication [7]. There is limited data on treatment adherence of HF patients in Africa, however, with most adherence data coming from western countries [4]. Due to the significant mortality rate of HF patients from Africa and the limited adherence data from this region, obtaining good adherence data are critical to help close the gap in understanding the high mortality rate. The method developed here forms an important part of a clinical study aimed at determining the treatment adherence of African HF patients.
Carvedilol is a non-selective β-blocking agent, acting as a competitive antagonist against β1-, β2- and α1 adrenergic receptors [8, 9]. Enalapril and perindopril are prodrugs of enalaprilat and perindoprilat, respectively, which are angiotensin-converting enzyme inhibitors (ACE-I) [8]. Carvedilol, in conjunction with an ACE-I, is often used in the treatment of HF [12]. Quantifying carvedilol, and the active ACE-I metabolites enalaprilat, and perindoprilat in human plasma is a direct approach to measuring the adherence of HF patients.
Van der Nagel et al. [13] reported an UPLC-MS/MS method for the simultaneous quantification of 8 antihypertensive drugs, including enalaprilat and perindoprilat. The authors used simple protein precipitation with a methanol and acetonitrile mixture, chromatography on a Acquity UPLC BEH C18 reversed phase column (100 mm × 2.1 mm, 1.7 μm) with a gradient mobile phase consisting of formic acid in water and methanol [13]. Lwin et al. [14] described a LC–MS/MS method for the detection of perindopril and perindoprilat with the use of a Kinetex C18 (100 mm × 2.1 mm, 1.7 μm) HPLC column and a gradient mobile phase consisting of methanol, water and formic acid. In this method, the positive ion mode was used for perindopril, while the negative ion mode was used for perindoprilat. The authors performed direct spiking of stock and internal standard solutions followed by precipitation with acetonitrile and ultracentrifugation to obtain a calibration range of 0.1–200 ng/mL [14]. Several publications describing LC–MS assays for carvedilol have been reported. Gomez et al. used a Hypurity C18, (50 mm × 4.6 mm, 5 µm) column with a mobile phase of acetonitrile and a 5 mM ammonium formate buffer with pH adjusted to 3 using formic acid (70:30, v/v). Solid phase extraction with Strata X columns was used for sample cleanup followed by LC–MS/MS detection in the positive ion mode over a calibration range of 0.5–500 ng/mL [15]. Other authors have performed a liquid–liquid extraction using phosphate buffer (pH9) and methyl-tert butyl ether followed by chromatographic separation on an Atlantis HILIC Silica column (50 mm × 3 mm, 5 µm) with a mobile phase consisting of acetonitrile and ammonium formate over a calibration range of 0.1–200 ng/mL [16].
Although different LC–MS/MS methods have been published for the quantification of carvedilol, enalaprilat, and perindoprilat, to date no method has been published for the simultaneous quantification of all three. A method for the determination of carvedilol, enalaprilat, and perindoprilat from 50 µL human plasma using protein precipitation and LC–MS/MS is described here. The plasma concentrations of carvedilol, enalaprilat or perindoprilat can be quantified for patients on either the carvedilol and enalapril, or carvedilol and perindopril medication regimens using the single analytical method presented here. The method developed is sensitive and requires a short analysis time, with the validated method successfully applied to the quantitative analysis of carvedilol, enalaprilat, and perindoprilat in samples received from a pilot clinical study.
The eventual aim is to assess adherence of HF patients from Africa who have been prescribed carvedilol, enalapril, and/or perindopril. The fact that samples being quantified for carvedilol, enalaprilat and perindoprilat can be quantified together with a single method, means the streamlining of instrument time and a reduction of chemical costs. Samples do not have to be separated according to the specific medication that the patient has taken, with cumulative time and cost savings over a long period having the potential to be significant. This is especially so if samples are being quantified on a large scale, such as during a clinical study.
Experimental
Collection and Storage of Samples
Donated plasma, free of carvedilol, enalaprilat, and perindoprilat was used during method development and for the preparation of calibration standards (STDs) and quality control samples (QCs), as well as during the validation experiments.
Chemicals
Carvedilol, enalaprilat dihydrate, and perindoprilat powder (Toronto Research Chemicals Inc., Toronto, Canada) were used as reference standards. Carvedilol-d5, enalaprilat-d5 sodium salt, and perindoprilat-13C3 (Toronto Research Chemicals Inc., Toronto, Canada) were utilised as internal standards. Ammonium acetate (≥ 99.99%) was supplied by Sigma-Aldrich (Modderfontein, South Africa) and LC–MS grade acetonitrile and methanol were purchased from Anatech (Bellville, South Africa) and Honeywell (B&J), respectively. Proanalyis grade acetic acid was obtained from Labchem (Johannesburg, South Africa). Deionized water was prepared using a Synergy Water Purification System purchased from Merck Millipore (Billerica, MA, USA).
Extraction Procedure
Plasma samples, including calibration standards, quality control samples, blanks, and unknown samples, stored at − 80 °C, were thawed and briefly vortexed at room temperature. Fifty microlitres of plasma was aliquoted into microcentrifuge tubes to which 200 µL of precipitation solution (methanol: acetonitrile (1:1, v/v) containing internal standard (2.41 ng/mL of Carvedilol-d5, 9.56 ng/mL of Enalaprilat-d5, and 4.78 ng/mL of Perindoprilat-13C3) was added. Samples were vortex-mixed for 30 s and left on bench to equilibrate for 5 min. Samples were then centrifuged at 20238g for 5 min and the supernatant transferred to glass tubes and evaporated under a gentle stream of nitrogen at approximately 40 °C for ~ 15 min. Once dried, 200 µL of reconstitution solution (methanol:water:formic acid (40:60:0.2, v/v/v)) was added and samples were vortexed for 30 s. Extracts were transferred to 96-well plates and placed in the LC–MS/MS autosampler at ~ 8 °C, with 20 µL of sample being injected.
LC–MS/MS Equipment and Conditions
An AB Sciex API 5500 (AB Sciex™, Germany) Qtrap mass spectrometer was used for detection employing electrospray ionization (ESI) in the positive ionisation mode. Mass spectrometer parameters were optimised by performing product ion scans of each analyte and the internal standards. The nebuliser gas, turbo gas, and curtain gas were set at 55, 55, and 30 psi, respectively, with the collision gas parameter set at the “medium” level. The source temperature was set at 500 °C and the ion spray voltage at 5500 V. The dwell times (ms) were optimised to 125, 35, 65, 105, 50, 60 for carvedilol, enalaprilat, perindoprilat, carvedilol-d5, enalaprilat-d5 and perindoprilat-13C3, respectively. The corresponding mass transitions were 407.1 > 100.1, 349.1 > 206.1, 341.2 > 98.1, 412.1 > 105.1, 354.1 > 211.0, and 344.2 > 100.1 for carvedilol, enalaprilat, perindoprilat, carvedilol-d5, enalaprilat-d5, and perindoprilat-13C3, respectively. Analyst Version 1.7.1(AB Sciex™, Germany) software was used to collect and analyse the data.
Chromatographic separation was obtained on a Restek Ultra Biphenyl column (100 mm × 2.1 mm, 3 µm). An Agilent 1260 Infinity II binary pump introduced the mobile phase while an Agilent 1200 Autosampler (Agilent, CA, USA) was used for sample injection. The pump was operated at a flow rate of 300 µL/min with the autosampler temperature and column temperature being set at ~ 8 °C and ~ 40 °C, respectively. The mobile phase consisted of 5 mM ammonium acetate and 0.1% acetic acid in water (mobile phase A), with mobile phase B consisting of 5 mM ammonium acetate and 0.1% acetic acid in water:methanol:acetonitrile (10:20:70, v/v/v). The chromatographic separation was achieved using a 7-min gradient elution. Gradient elution started with the initial mobile phase composition at 5% of B, and then linearly increased to 90% within 0.5 min at which percentage it was held constant for 2.5 min. Mobile phase B was then decreased to 5% in 0.1 min followed by a 3.4-min equilibration before the next injection.
Method Validation
The method was validated according to the 2018 US Food and Drug Administration (FDA) specific for industry bioanalytical method validation guidelines [17].
Calibration Standard, Quality Control, Internal Standard and Dilution Quality Control Preparation
Initial stock solutions of carvedilol, enalaprilat, perindoprilat, carvedilol-d5, and perindoprilat-13C3 with a concentration of 1000 µg/mL and enalaprilat-d5 with a concentration of 5000 µg/mL were prepared in methanol and stored at ~ − 80 °C. Two sets of working solutions were prepared; one used for calibration standard (STD) preparation and one for quality control (QC) preparation. For the STDs, a working solution containing the three cardiovascular drugs was prepared by spiking 20 µL of each of the stock solutions of enalaprilat, perindoprilat, and carvedilol into 2.44-mL methanol. For the QCs, a working solution containing the three cardiovascular drugs was prepared by spiking 20 µL of each 1 mg/mL stock solution of enalaprilat, perindoprilat and carvedilol into 4.94-mL methanol. From these solutions, a series of working solutions was prepared volumetrically in methanol.
Freshly spiked STDs were assayed in each analytical run by spiking blank tripotassium ethylenediaminetetraacetic acid (K3EDTA) plasma (1.9 mL) with working solutions to cover the calibration range of 0.200–200 ng/mL.
Similarly, QCs were prepared by spiking blank K3EDTA plasma (1.9 mL) with working solutions to obtain final concentrations of 0.200 (LLOQ), 0.550 (low), 80.0 (medium) and 160 (high) ng/mL.
The calibration range was validated by analysing the QC samples in six-fold at the four concentration levels over a 3-day period to determine the intra- and inter-day accuracy and precision. To determine if samples originally reported as ALQ (above the upper limit of the standard curve) may be diluted to within the calibration range with accuracy and precision, six extra high QC Dilute samples were prepared at a concentration two times higher than the ULOQ (i.e., 400 ng/mL) for carvedilol, enalaprilat, and perindoprilat. These were then diluted 1:4 (v/v) with blank plasma. The calibration curve fits a quadratic regression (weighted by 1/x for carvedilol and 1/x2 for enalaprilat and perindoprilat) over the range 0.2 to 200 ng/mL.
Stock Solution and Working Solution Stability
Stock solution stability for all analytes and internal standards was evaluated against freshly prepared reference solutions. Stability was assessed at ~ − 80 °C over 62 days and at room temperature for ~ 6 h, diluting 10 µL of each test stock solution with 1 mL of methanol and comparing the absorbance of three separate samples against freshly prepared samples. UV absorbances at 242 nm for carvedilol and 206 nm for enalaprilat and perindoprilat were used.
Working solution stability was assessed for each of the analytes by diluting the test and reference working solutions in injection solution containing the respective internal standards. This was evaluated over 7 days at ~ − 80 °C and for ~ 4 h at room temperature. The resulting peak area ratios were compared by means of LC–MS/MS.
On-instrument stability and Reinjection Reproducibility
Reinjection reproducibility was evaluated by reinjecting extracted samples which had remained in the autosampler for 48 h at ~ 8 °C. Autosampler stability was assessed by comparing low (0.550 ng/mL) and high (160 ng/mL) quality control peak area ratios (in six-fold) to those obtained during the first injection. This allowed for an estimation of absolute autosampler stability over 48 h, demonstrating the suitability of the internal standard(s) to track the stability of the analyte(s).
Stability in Matrix
Stored quality control samples containing carvedilol, enalaprilat, and perindoprilat (~ − 20 °C for 15 days) were analysed against a freshly prepared calibration curve to determine the stability of carvedilol, enalaprilat, and perindoprilat in matrix at − 20 °C as well as − 80 °C.
Freeze–Thaw Stability
To ascertain freeze–thaw stability, low and high quality controls were prepared and frozen at ~ − 80 °C before being put through three consecutive freeze–thaw cycles. Sample aliquots were frozen for at least 24 h prior to starting this experiment. Each cycle consisted of sufficient thawing time at room temperature followed by 12–24 h freezing time. These samples were analysed against a freshly prepared valid calibration curve and assessed for accuracy against the nominal QC concentration.
Bench-Top Stability
To ascertain bench-top stability, low and high quality controls were frozen at ~ − 80 °C, and subsequently left on the bench for approximately 6 h to thaw unaided (maximum anticipated time that future study samples will be left thawed until extracted). These samples were analysed against a valid freshly prepared calibration curve.
Recovery
Recovery was assessed by extracting quality controls in six different lots of matrix (test samples). These extracts were compared to the same set of matrix samples, extracted, and then spiked post extraction (reference samples). Recovery is calculated by comparing the analyte peak areas observed in the test samples vs. those observed in the reference samples. This is expressed as percentage recovery.
Process Efficiency
Process efficiency was evaluated by comparing the results from quality controls at the high, medium, and low concentrations (160, 80, and 0.550 ng/mL, respectively) to neat, un-extracted samples prepared in mobile phase B ([water:methanol:acetonitrile, (10:20:70,v/v/v)], with 5 mM ammonium acetate and 0.1% acetic acid), with appropriate adjustments made for dilution during the extraction process. Internal standard concentration was reflective of the working concentration of the method. The ratio of the analyte peak areas after extraction to those of the neat samples were then expressed as the percentage process efficiency.
Matrix Effect
The suppressive or enhancive effects of the biological matrix (plasma) was evaluated according to methodology set out by Matuszewski et al. [18]. The peak areas of the analytes spiked into plasma after extraction were expressed as a fraction of the peak areas observed in neat solution and used to calculate the observed matrix effects and to assess whether the effects observed affect the results of the assay.
Haemolysis Evaluation
The influence of the presence of 2% haemolysed blood was assessed by assaying six haemolysed samples at high and low concentrations. The analyte to internal standard response ratios were compared to those observed following the assay of six normal human plasma samples at the same high and low concentrations.
Whole Blood Stability
Stability of the analytes in whole blood was assessed under the appropriate conditions to estimate the maximum amount of time that the whole blood sample may be left at room temperature before being centrifuged to obtain plasma (i.e., to cover the stability during sample collection). Stability in whole blood was tested after 2 h at room temperature. Fresh whole blood was spiked at both low and high QC levels. Reference samples were equilibrated and immediately centrifuged for plasma collection and then stored at ~ − 80 °C. The remaining test samples were centrifuged after 2 h at room temperature and the plasma was then stored at ~ − 80 °C until analysis. The stability was evaluated by comparing the peak area ratios for the test samples to those of the reference samples.
Specificity and Carryover
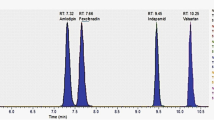
Six blank plasma sources were assayed without internal standard and the chromatograms reviewed to ensure that the bioanalytical method was able to differentiate and quantify carvedilol, enalaprilat and perindoprilat in the presence of other endogenous matrix components in the sample. A double blank sample was positioned in the injection sequence immediately after the highest calibration standard to assess possible carryover effects.
Sensitivity
The signal-to-noise ratio (S/N) was calculated to determine the analyte response at the lowest limit of quantification (LLOQ). The mean (S/N) should be greater than 5 at the LLOQ [17].
Cross Talk
The potential for cross talk between the analyte and internal standard MRM channels was evaluated. Due to the method being multiplexed, each analyte was spiked at the upper limit of quantification and assayed to see if it appears in the other analyte channels as well. Contribution from the prodrugs as well as the perindopril metabolite, perindoprilat glucuronide, was also evaluated. The contribution of the analyte to the internal standard signal was assessed by assaying the ULOQ sample and examining the ISTD channel at the appropriate retention time. The contribution of each analyte was assessed.
Clinical Application of the Method
The validated assay was used to analyse clinical samples which had been collected during a pilot study. The University of Cape Town Faculty of Health Science Research Ethics Committee gave approval to conduct this study (HREC/REF: 480/2018). Each participant provided written informed consent.
Blood samples were taken from ten participants (four withdrew during the course of the pilot study) on combined enalapril and carvedilol treatment at steady state concentrations. Samples were taken pre-dose (immediately before the dose was administered) and at 1.5, 3, 5, 8, and 12 h after the dose was administered. Participants were either on 20 mg/day or 10 mg/day enalapril (taken twice daily) with carvedilol dosages being 25 mg/day or 50 mg/day (taken twice daily). After sampling, participants were then switched to perindopril instead of enalapril with an 8 mg/day dosage for those participants on 20 mg/day enalapril and 4 mg/day for those participants on 10 mg/day enalapril. Blood samples were taken 4 weeks after the switch for carvedilol and perindoprilat, with samples taken pre-dose as well as 1.5, 3, 5, 8, 12, and 24 h after the dose was administered.
Results and Discussion
An efficient, multiplex extraction method was developed using protein precipitation. It was found that a mixture of acetonitrile and methanol (1:1, v/v) resulted in satisfactory extraction. A reverse phase gradient method where the organic mobile phase B was adjusted from 5 to 90% in 30 s, was utilised resulting in corresponding retention times at approximately 4.1, 3.0, 3.1, 4.1, 3.0, and 3.1 min for carvedilol, enalaprilat, perindoprilat, carvedilol-d5, enalaprilat-d5, and perindoprilat-13C3, respectively. The full scan positive ion mass spectra indicated that carvedilol, enalaprilat, and perindoprilat were protonated molecular ions of m/z 407.1, m/z 349.1, and m/z 341.2, respectively. The chosen MRM transitions are summarised in Table 1, with proposed fragmentations shown in Fig. 1.
Final product ion mass spectra with proposed fragmentations of a carvedilol, b enalaprilat, c perindoprilat, d carvedilol-d5, e enalaprilat-d5, and f perindoprilat-13C3. (Table 1 is a summary of quantifiers and qualifiers used.)
During validation, the assay was shown to produce accurate concentrations over three consecutive and independent runs with percentage accuracies (%Nom) for carvedilol, enalaprilat, and perindoprilat, ranging from 93.7% to 108.4% and 94.6% to 105.1% for quality controls and standards, respectively. The corresponding precision (CV%) statistics were all below 12.5%. The calibration curves fit quadratic (weighted by 1/x for carvedilol and 1/x2 for enalaprilat and perindoprilat, x = concentration) regressions over the range of 0.200–200 ng/mL for all analytes. The combined 3-day summary of accuracy and precision data obtained during the validation is shown in Table 2.
Stability data is presented in Tables 2 and 3. Carvedilol and perindoprilat stock solutions were found to be stable for ~ 6 h at room temperature in methanol, with enalaprilat demonstrating ~ 24 h stability in methanol at room temperature. All three internal standards demonstrated ~ 24 h stability in methanol at room temperature. The long-term stock solution stability assessment for carvedilol, enalaprilat, and perindoprilat showed the analytes to be stable for at least 62 days in methanol at ~ − 80 °C.Working solutions were found to be stable at room temperature at both the highest working solution concentration (4.00 µg/mL) and the lowest working solution concentration (0.0040 µg/mL) for ~ 4 h. Long-term working solution stability was demonstrated at ~ − 80 °C for a period of up to ~ 7 days in methanol (Table 3).
Reinjection reproducibility and on-instrument stability for carvedilol, enalaprilat and perindoprilat were assessed to evaluate consistency in instrument performance and analyte stability should instrument failure occur. The accuracy was between 93 and 110% and precision was all less than 11%. Carvedilol and enalaprilat extracts demonstrated ~ 24 h on-instrument stability, with perindoprilat extracts exhibiting stability for ~ 48 h. Enalaprilat and carvedilol extracts can therefore be reinjected within 24 h if there is an instrument failure. For perindoprilat, extracts can be reinjected within 48 h.
Matrix stability results indicate stability of carvedilol, enalaprilat, and perindoprilat when stored at ~ − 80 °C and ~ − 20 °C for at least 15 days (Table 2). Freeze–thaw stability was demonstrated following three cycles of thawing at room temperature and storage at ~ − 80 °C only. Concentrations observed for freeze–thaw QCs tested were all within 9% of the nominal concentrations. Bench top stability percentage differences across high and low concentrations were all within 10% of the nominal concentrations, indicating stability in plasma for ~ 6 h at room temperature (Table 2).
The average process efficiency of the method for carvedilol, enalaprilat, and perindoprilat was 68.2%, 77.0%, and 75.2%, respectively (%CV = 2.9, 5.1, 1.3, respectively). The corresponding average recovery over the high, medium, and low concentrations of carvedilol, enalaprilat, and perindoprilat was determined to be 72.9, 77.1, and 77.0 (%CV = 7.8, 2.9, and 0.9, respectively). Using the criteria as outlined by Matuszewski et al. [18], no significant endogenous matrix effects were observed (Table 4). The three analytes were also shown to be stable in whole blood for a period of up to 2 h at room temperature. In addition, quantification of carvedilol, enalaprilat, and perindoprilat was shown not to be affected by the presence of up to 2% haemolysed blood.
Significant carry-over was observed for all three analytes, with perindoprilat being particularly problematic. This was mitigated by inserting blank extracted matrix samples between each sample during analysis. The observed signal-to-noise ratio at the LLOQ for all three analytes was in excess of 50, far exceeding the accepted criterion of 5[17]. The chromatograms of blank extracted samples as well as extracted LLOQ spiked samples appear in Fig. 2. Technical cross talk was not observed but the in-source formation of perindoprilat and enalaprilat did occur during perindopril, perindoprilat-glucuronide and enalapril sample injection. However, due to the peaks eluting separately, independent integration and quantification can be done, as a result this contribution is not of consequence.
Application of the Method to a Clinical Study
The validated method was applied successfully to a pharmacokinetic pilot study. The study allowed to evaluate the robustness of the method developed using actual patient samples; with the pharmacokinetic data allowing a wide concentration range to be observed. The baseline characteristics of the participants are listed in Table 5. The assay was sufficiently sensitive such that plasma concentrations were quantifiable over the entire dosage interval (12 h for carvedilol and enalaprilat, and 24 h for perindoprilat). It must be noted that most of the patients taking part in the study were clinically obese (Table 5). Obesity can have an influence on the absorption, distribution, metabolism, and clearance of a drug when compared to that of an individual of normal weight [19, 20]. This fact, along with the small sample size, must be considered when observing deviations from expected data, such as a non-linear relationship between dose and plasma concentrations.
Plasma concentration vs. time profiles are depicted in Fig. 3. The bidaily dosage for carvedilol was 12.5 mg in three participants and 25 mg in three participants. The time to peak concentration (1.5 h) is similar to that observed for previous studies [8, 21]. Plasma concentrations did not display a proportional increase with dose as seen with previous studies [21], with peak steady state concentrations similar (approximately 50.0 ng/mL) for both the 12.5-mg bidaily dose group and the 25 mg bidaily dose group.
Semi-log plots of mean a carvedilol, b enalaprilat, and c perindoprilat plasma concentrations versus time in human volunteers. Three indviduals were on the 25-mg BD carvedilol dose, three on the 12.5-mg BD dose, five on the 10-mg BD enalapril dose, one on the 5-mg enalapril BD dose, five on the 8-mg perindopril dose and one on the 4-mg perindopril dose. The error bars represent the standard deviation of the mean concentrations of the individuals on the different dosages
Five participants were on the 10-mg bidaily enalapril dose regimen with one participant on the 5-mg bidaily dose regimen. Enalaprilat plasma concentrations peaked 3 h after oral administration of enalapril, corresponding with literature [22, 23]. The mean peak enalaprilat plasma concentration observed for the 10-mg bidaily group was approximately 63.0 ng/mL, with the participant on the 5-mg bidaily dose having a peak concentration of around 53.0 ng/mL. Steady state data for enalaprilat at the indicated enalapril dosages and dosage times could not be found for comparison in the literature. A single dose of 10-mg enalapril, however, resulted in a peak enalaprilat concentration of approximately 55 ng/mL [23, 24].
For perindopril, five participants were on the 8 mg/day dose with one participant on the 4 mg/day dose. Peak perindoprilat plasma concentrations were observed 3 h after the oral administration of perindopril; in line with that in the literature [25]. Peak perindoprilat plasma concentrations observed were approximately 23.0 ng/mL and 18.0 ng/mL for the 8 mg/day and 4 mg/day doses respectively with these peaks falling within the peak concentration ranges seen at steady state in literature [25]. Both enalaprilat and perindoprilat display biphasic elimination, consisting of a short initial elimination half-life followed by a prolonged phase with a half-life of more than 30 h [23, 26, 27], the latter can be exploited for adherence monitoring purposes.
Conclusions
A novel, sensitive and specific multiplex LC–MS/MS assay to determine carvedilol, enalaprilat and perindoprilat concentrations in human plasma was developed, fully validated, and subsequently applied to a pilot pharmacokinetic study to study the oral administration of carvedilol, enalapril, and perindopril. The assay may be used for the therapeutic drug monitoring of carvedilol and the active metabolites of enalapril and perindopril; enalaprilat and perindoprilat, respectively, which are often used as beta blocker and ace-inhibitor combinations for the treatment of hypertension and heart failure [12].
The validation data presented prove that the method developed is not only suited for single dose analysis, but also multiple dose pharmacokinetic studies and clinical trial samples. The method is therefore well suited for quantifying carvedilol, enalaprilat and perindoprilat to monitor adherence in patients prescribed carvedilol, enalapril and perindopril, suffering from heart failure.
References
Ferreira JP, Kraus S, Mitchell S, Perel P, Piñeiro D, Chioncel O, Colque R, de Boer R, Gomez-Mesa JE, Grancelli H, Lam CSP, Martinez-Rubio A, McMurray JJV, Mebazaa A, Panjrath G, Piña IL, Sani M, Sim D, Walsh M, Yancy C, Zannad F, Sliwa K (2019) World heart federation roadmap for heart failure. Glob Heart 14:197–214. https://doi.org/10.1016/j.gheart.2019.07.004
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes-Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen M-L, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen J-C, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa-Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK (2021) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021:1–128. https://doi.org/10.1093/eurheartj/ehab368
Ajayi AA, Sofowora GG, Ladipo GO (2020) Explaining heart failure hyper-mortality in sub saharan africa: global genomic and environmental contribution review. J Natl Med Assoc 112:141–157. https://doi.org/10.1016/j.jnma.2020.02.003
Ruf V, Stewart S, Pretorius S, Kubheka M, Lautenschläger C, Presek P, Sliwa K (2010) Medication adherence, self-care behaviour and knowledge on heart failure in urban South Africa: the Heart of Soweto study., Cardiovasc. J Afr 21:86–92
Hood SR, Giazzon AJ, Seamon G, Lane KA, Wang J, Eckert GJ, Tu W, Murray MD (2018) Association Between Medication Adherence and the Outcomes of Heart Failure. Pharmacother J Hum Pharmacol Drug Ther. https://doi.org/10.1002/phar.2107
Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM (2016) Medication Adherence Interventions Improve Heart Failure Mortality and Readmission Rates: Systematic Review and Meta-Analysis of Controlled Trials. J Amercian Hear Assoc 10:1–18. https://doi.org/10.1161/JAHA.115.002606
D. Shah, K. Simms, D. Barksdale, J.-R. Wu, Improving medication adherence of patients with chronic heart failure: challenges and solutions, Res. Reports Clin. Cardiol. (2015) 87. https://doi.org/10.2147/rrcc.s50658.
Do Carmo Borges NC, Mendes GD, De Oliveira Silva D, Rezende VM, Barrientos-Astigarraga RE, De Nucci G (2005) Quantification of carvedilol in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Application to bioequivalence study. J Chromatogr B Anal Technol Biomed Life Sci 822:253–262. https://doi.org/10.1016/j.jchromb.2005.06.012
Henderson LS, Tenero DM, Baidoo CA, Campanile AM, Harter AH, Boyle D, Danoff TM (2006) Pharmacokinetic and pharmacodynamic comparison of controlled-release carvedilol and immediate-release carvedilol at steady state in patients with hypertension. Am J Cardiol 98:17–26. https://doi.org/10.1016/j.amjcard.2006.07.015
Dinicolantonio JJ, Lavie CJ, O’Keefe JH (2013) Not all angiotensin-converting enzyme inhibitors are equal: focus on ramipril and perindopril. Postgrad Med 125:154–168. https://doi.org/10.3810/pgm.2013.07.2687
Portoles A, Terleira A, Almeida S, Garcia-Arenillas M, Caturla M-C, Filipe A, Vargas E (2004) Bioequivalence study of two formulations of enalapril, at a single oral dose of 20 mg., Curr. Ther Res 65: 34–46. http://cat.inist.fr/?aModele=afficheN&cpsidt=15644465.
Remme WJ, Riegger G, Hildebrandt P, Komajda M, Jaarsma W, Bobbio M, Soler-Soler J, Scherhag A, Lutiger B, Rydén L (2004) The benefits of early combination treatment of carvedilol and an ACE-inhibitor in mild heart failure and left ventricular systolic dysfunction. The carvedilol and ACE-inhibitor remodelling mild heart failure evaluation trial (CARMEN). Cardiovasc Drugs Ther 18:57–66. https://doi.org/10.1023/B:CARD.0000025756.32499.6f
van der Nagel BCH, Versmissen J, Bahmany S, van Gelder T, Koch BCP (2017) High-throughput quantification of 8 antihypertensive drugs and active metabolites in human plasma using UPLC–MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 1060:367–373. https://doi.org/10.1016/j.jchromb.2017.06.029
Lwin EMP, Gerber C, Song Y, Leggett C, Ritchie U, Turner S, Garg S (2017) A new LC-MS/MS bioanalytical method for perindopril and perindoprilat in human plasma and milk. Anal Bioanal Chem 409:6141–6148. https://doi.org/10.1007/s00216-017-0552-y
Gomes NA, Vaidya VV, Pudage AM, Joshi SS, Parekh SA, Tamhankar AV (2009) Application of a sensitive, rapid and validated LC-MS-MS method for the determination of carvedilol in human plasma. Chromatographia 69:19–25. https://doi.org/10.1365/s10337-008-0848-3
Jeong DW, Kim YH, Ji HY, Youn YS, Lee KC, Lee HS (2007) Analysis of carvedilol in human plasma using hydrophilic interaction liquid chromatography with tandem mass spectrometry. J Pharm Biomed Anal 44:547–552. https://doi.org/10.1016/j.jpba.2006.12.017
FDA, Bioanalytical Method Validation Guidance, (n.d.). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry.
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030. https://doi.org/10.1021/ac020361s
Hanley MJ, Abernethy DR, Greenblatt DJ (2010) Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 49:71–87. https://doi.org/10.2165/11318100-000000000-00000
De Baerdemaeker LEC, Mortier EP, Struys MMRF (2004) Pharmacokinetics in obese patients. Contin Educ Anaesthesia Crit. Care Pain 4:152–155. https://doi.org/10.1093/bjaceaccp/mkh042
D. Tenero, S. Boike, D. Boyle, B. Ilson, H.F. Fesniak, S. Brozena, D. Jorkasky, Steady-State Pharmacokinetics of Carvedilol and Its Enantiomers in Patients with Congestive Heart Failure, (n.d.). Doi: https://doi.org/10.1177/00912700022009576.
MacFadyen HL, Meredith RJ, Elliott PA (1993) Enalapril clinical pharmacokinetics and pharmacokinetic-pharmacodynamic relationships. Clin Pharmacokinet 25:274–282. https://doi.org/10.1055/b-0034-75318
Smeets NJL, Schreuder MF, Dalinghaus M, Male C, Lagler FB, Walsh J, Laer S, de Wildt SN (2020) Pharmacology of enalapril in children: a review. Drug Discov Today 25:1957–1970. https://doi.org/10.1016/j.drudis.2020.08.005
Arafat T, Awad R, Hamad M, Azzam R, Al-Nasan A, Jehanli A, Matalka K (2005) Pharmacokinetics and pharmacodynamics profiles of enalapril maleate in healthy volunteers following determination of enalapril and enalaprilat by two specific enzyme immunoassays. J Clin Pharm Ther 30:319–328. https://doi.org/10.1111/j.1365-2710.2005.00646.x
Louis B, Workman WJ, Conway BS, Worland EL, Rowley P, Drummer K, Mcneil O, Harries JJ, Jarrot G (1992) Single-dose and steady-state pharmacokinetics and pharmacodynamics of perindopril in hypertensive subjects. J Cardiovasc Pharmacol 20:505–511
Sennesael J, Ali A, Sweny P, Vandenburg M, Slovic D, Dratwa M, Resplandy G, Genissel P, Desche P (1992) The pharmacokinetics of perindopril and its effects on serum angiotensin converting enzyme activity in hypertensive patients with chronic renal failure. Br J Clin Pharmacol 33:93–99. https://doi.org/10.1111/j.1365-2125.1992.tb04006.x
Critchley J, Tomlinson B, Resplandy G (1995) Comparison of the pharmacokinetics and pharmacodynamics of oral doses of perindopril in normotensive Chinese and Caucasian volunteers. Br J Clin Pharmacol 39:361–368. https://doi.org/10.1111/j.1365-2125.1995.tb04463.x
Funding
This work was supported by the Hippocrate foundation, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joubert, A., Kellermann, T., Joubert, A. et al. Simultaneous Determination of Carvedilol, Enalaprilat, and Perindoprilat in Human Plasma Using LC–MS/MS and Its Application to a Pharmacokinetic Pilot Study. Chromatographia 85, 455–468 (2022). https://doi.org/10.1007/s10337-022-04154-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04154-y