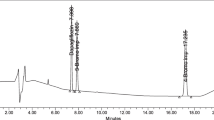
Abstract
Ipragliflozin degradation behavior was studied under different conditions: acidic, basic, photolytic, oxidative and thermal degradation conditions. This forced degradation study showed the extensive degradation of Ipragliflozin under acidic, basic and oxidative conditions while showed high stability under thermal and photo-degradation conditions. The separation of Ipragliflozin and its degradation products was done using Hypersil Gold® UPLC C18 column with 1.9 μm particle size (3 × 50 mm) as stationary phase and a mobile phase composed of acetonitrile: potassium monobasic phosphate buffer pH 3 (50:50; v/v) delivered at flow rate of 0.6 mL min−1. Validation of the proposed method was carried out in accordance to the International Council for Harmonisation’s guidelines. The method was found to be linear within the concentration range 5.0–50.0 µg mL−1 with a limit of detection of 1.48 µg mL−1. Accuracy was proven as the percentage recovery was 98.57 ± 0.40 and the percentage relative standard deviation was 0.82 which ascertained the precision. After chromatographic separation, mass characterization was used to structurally elucidate the degradation products and to propose the degradation pathway. Kinetics parameters of oxidative, acidic and basic degradation processes were determined and it was demonstrated that the degradation appears to follow a pseudo-first-order reaction. The simplicity and sensitivity of the proposed method promote its regular use in quality control laboratories.
Graphical Abstract











Similar content being viewed by others
Availability of data and material
All data generated or analysed during this study are included in this published article.
Code availability
Not applicable.
References
Wright EM (2001) Renal Na+-glucose cotransporters. Am J Physiol Ren Physiol 280:F10–F18. https://doi.org/10.1152/ajprenal.2001.280.1.f10
Lee YJ, Lee YJ, Han HJ (2007) Regulatory mechanisms of Na+/glucose cotransporters in renal proximal tubule cells. Kidney Int 72:S27-35. https://doi.org/10.1038/sj.ki.5002383
Hummel CS, Lu C, Loo DDF et al (2011) Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300:C14-21. https://doi.org/10.1152/ajpcell.00388.2010
Kalra S (2014) Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther 5:355–366. https://doi.org/10.1007/s13300-014-0089-4
Nauck MA (2014) Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther 8:1335–1351. https://doi.org/10.2147/DDDT.S50773
Tahara A, Kurosaki E, Yokono M et al (2013) Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol 715:246–255. https://doi.org/10.1016/j.ejphar.2013.05.014
Tahara A, Kurosaki E, Yokono M et al (2012) Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol 385:423–436. https://doi.org/10.1007/s00210-011-0713-z
Salama FM, Attia KA, Abouserie A et al (2018) Stability-indicating HPLC-DAD method for the determination of ipragliflozine. Innoriginal Int J Sci 5:11–15
Kim NS, Kim KY, Yoo GJ et al (2018) Determination of 26 anti-diabetic compounds in dietary supplements using a validated UPLC method. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 35:387–394. https://doi.org/10.1080/19440049.2017.1332429
Kobuchi S, Ito Y, Yano K, Sakaeda T (2015) A quantitative LC–MS/MS method for determining ipragliflozin, a sodium-glucose co-transporter 2 (SGLT-2) inhibitor, and its application to a pharmacokinetic study in rats. J Chromatogr B 1000:22–28. https://doi.org/10.1016/j.jchromb.2015.07.013
Kadokura T, Zhang W, Krauwinkel W et al (2014) Clinical pharmacokinetics and pharmacodynamics of the novel SGLT2 inhibitor ipragliflozin. Clin Pharmacokinet 53:975–988
Lam YH, Leung MT, Ching CK, Mak TWL (2020) Simultaneous detection of 24 oral antidiabetic drugs and their metabolites in urine by liquid chromatography—tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 1141:122020. https://doi.org/10.1016/j.jchromb.2020.122020
Salama FM, Attia KA, Abouserie AA et al (2018) Spectrophotometric Processing Data For Determination Ipragliflozine In Pure And In Tablet Dosage Form. Asian J Pharm Heal Sci 8:1894–1901
Elhassan MM, Mahmoud AM, Hegazy MA, Mowaka S (2021) Electrochemical determination of ipragliflozin in pure form and in spiked human plasma on a glassy carbon electrode. J Electrochem Soc 168:036507. https://doi.org/10.1149/1945-7111/abe511
Blessy M, Patel RD, Prajapati PN, Agrawal YK (2014) Development of forced degradation and stability indicating studies of drugs—a review. J Pharm Anal 4:159–165
Nováková L, Solichová D, Solich P (2006) Advantages of ultra performance liquid chromatography over high-performance liquid chromatography: comparison of different analytical approaches during analysis of diclofenac gel. J Sep Sci 29:2433–2443. https://doi.org/10.1002/jssc.200600147
Moussa BA, Hashem HMA, Mahrouse MA, Mahmoud ST (2018) Experimental design approach in HPLC method development: application for the simultaneous determination of sacubitril and valsartan in presence of their impurities and investigation of degradation kinetics. Chromatographia 81:139–156. https://doi.org/10.1007/s10337-017-3425-9
Kurmi M, Sahu A, Singh S (2017) Stability behaviour of antiretroviral drugs and their combinations. 5: Characterization of novel degradation products of abacavir sulfate by mass and nuclear magnetic resonance spectrometry. J Pharm Biomed Anal 134:372–384. https://doi.org/10.1016/j.jpba.2016.10.019
Lambropoulou D, Evgenidou E, Saliverou V et al (2017) Degradation of venlafaxine using TiO2/UV process: kinetic studies, RSM optimization, identification of transformation products and toxicity evaluation. J Hazard Mater 323:513–526. https://doi.org/10.1016/j.jhazmat.2016.04.074
Kim HY, Kim TH, Cha SM, Yu S (2017) Degradation of sulfamethoxazole by ionizing radiation: Identification and characterization of radiolytic products. Chem Eng J 313:556–566. https://doi.org/10.1016/j.cej.2016.12.080
Loos G, Van Schepdael A, Cabooter D (2016) Quantitative mass spectrometry methods for pharmaceutical analysis. Philos Trans R Soc A Math Phys Eng Sci 374. 20150366
Devrukhakar PS, Shiva Shankar M, Shankar G, Srinivas R (2017) A stability-indicating LC–MS/MS method for zidovudine: Identification, characterization and toxicity prediction of two major acid degradation products. J Pharm Anal 7:231–236. https://doi.org/10.1016/j.jpha.2017.01.006
Can NÖ (2018) Development of validated and stability-indicating LC–DAD and LC–MS/MS methods for determination of avanafil in pharmaceutical preparations and identification of a novel degradation product by LCMS–IT–TOF. Molecules 23:1771. https://doi.org/10.3390/molecules23071771
ICH Harmonized Tripartite Guideline (1996) Validation of analytical procedures: text and methodology. Q2 (R1) (current step 4 version, Parent guidelines on methodology)
Food and Drug Administration, HHS (2003) In: International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use ICH harmonised tripartite guideline stability testing of new drug substances and products. Q1A (R2)
Zaghary WA, Mowaka S, Hendy MS (2019) Kinetic degradation study of dapagliflozin coupled with UHPLC separation in the presence of major degradation product and metformin. Chromatographia 82:777–789. https://doi.org/10.1007/s10337-019-03702-3
Acknowledgements
The authors are thankful to Associate Professor Bassem Naguib for his valuable contribution in explaining the proposed structures of the obtained degradation products within this work.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elhassan, M.M., Mahmoud, A.M., Hegazy, M.A. et al. Kinetic Degradation Study of Ipragliflozin Coupled with MS/MS Structural Elucidation. Chromatographia 85, 233–245 (2022). https://doi.org/10.1007/s10337-021-04127-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04127-7