Abstract
A wealth of physico-chemical information has been obtained on an insoluble and crystalline polypyrrole/titanium oxide (PPY/TiO2) using the Inverse Gas Chromatography Method (IGC). Twenty-five solvents (test compounds) with different specific interactions were used. PPY/TiO2 was synthesized and characterized by FTIR and DSC methods. The following characteristics of PPY/TiO2 were explored: changes in morphology, the strength of solvent–PPy/TiO2 interactions, dispersive surface energy, percent crystallinity, solvents and PPy/TiO2 solubility parameters, and molar heat of sorption, mixing and evaporation (\(\Delta H_{1}^{s}\), \(\Delta H_{1}^{\infty }\), \(\Delta H_{1}^{v}\)). Interaction parameters were corrected to be free from the solvents’ chemical nature. Nonane, propyl acetate, and methyl ethyl ketone showed stronger interactions and a higher affinity to PPy/TiO2; yet, none of these solvents can dissolve PPy/TiO2. IGC showed that PPy/TiO2 has 59.34 mJ/m2 dispersive surface energy at 100 °C and 98% crystalline at 60 °C. Comprehensive solvents and PPy/TiO2 solubility parameters were obtained and comparted with that of soluble and insoluble polymers.
Graphic abstract
Inverse Gas Chromatography (IGC) along with DSC and FTIR methods investigated the morphology, surface energy, degree of crystallinity and the thermodynamics of conducting polypyrrole/TiO2. The solubility parameters were calculated for 27 solvents.










Similar content being viewed by others
References
Duaij OK, Al-Ghamdi A, Al-Saigh ZA (2013) J Chromatogr A 1291:137–145
Duaij OK, Al-Ghamdi A, Al-Saigh ZA (2013) Polym Prepr 53:163
Wu R, Que D, Al-Saigh ZA (2007) J Chroma A 1146:93
Al-Ghamdi A, Al-Saigh ZY (2003) Polym Prepr 44:1045
Al-Ghamdi A, Al-Saigh ZY (2002) J Chromatogr A 969:229–243
Kanwala F, Siddiqib SA, Batoolb A, Imrana M, Mushtaqa W, Jamilc T (2001) Synth Met 161:335
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269–8285
Gao Y, Ding K, Xu X, Wang Z, Yu D (2014) RSC Adv. 4:27130
Nguyen TT, Duong NH (2016) J Nanomater 2016:1–6
Yu C, Li G, Kumar S, Kawasaki H, Jin R (2013) J Phys Chem Lett 4:2847–2852
Eisazadeh H (2008) World Appl Sci J 3:14
Chehimi MM, Abel ML, Ficker F, Delamar M, Jada A, Brown AM, Watts JF (1998) J Chim Phys 95:1282
Yang CY, Cao CY, Smith Y, Heeger P (1993) J Synth Meter 53:293
Stockton WB, Rubner MF (1994) Mater Res Soc Symp Proc 328:257
Zhong B, Al-Saigh ZY (2015) Eur Polym J 67:174
Al-Ghamdi A, Melibari M, Al-Saigh ZY (2006) J Appl Polym Sci 101:3076–3089
Zhong B, Al-Saigh ZY (2012) J Appl Polym Sci 123:2616–2627
Al-Ghamdi A, Melibari M, Al-Saigh ZY (2005) J Polym Environ 13:319–327
Al-Saigh ZY (2006) Characterization of biodegradable polymer blends by inverse gas chromatography: amylopectin and its blends. In: Khemami, Scholz (eds) Degradable polymers and materials principles and practice. ACS Symposium Series, vol. 939 Chapter 20
Al-Saigh ZY (1994) Polym News 19:269
Lipson JEG, Guillet JE (1982) J Coat Technol 54(684):89–93
Braun JM, Guillet JE (1976) Adv Polym Sci 21:107
Munk P (1991) Modern methods of polymer characterization. In: Barth H, Mays JW (eds) Chemical analysis: a series of monographs on analytical chemistry and its applications, Winefordner, J. D., vol 113. Wiley, New York, pp 151–200
Munk P, Hattam P, Abdual-Azim A, Du Q (1990) Makromol Chem, Macromol Symp 38:205
Hansen CM (1967) J Paint Technol 104:505
El-Hibri MJ, Cheng W, Munk P (1988) Macromolecules 21:3458
Al-Saigh ZY, Munk P (1984) Macromolecules 17:803
Al-Saigh ZY, Guillet J (2000) Inverse Gas Chromatography in Analysis of Polymers and Rubbers. In: Meyers R (ed) Encyclopedia of Analytical Chemistry: Instrumentation and Applications. John Wiley & Sons Ltd, Chichester, pp 7759–7792
AlSaigh ZY (1997) Int J Polym Charact Anal 3:249
Chehimi MM, Abel ML, Sahraoui Z, Fraoua K, Lascelles SF, Armes SP (1997) Int. J. Adhesion and Adhesives 17:1
Ustamehmetoglu B, Kizilcan N, Demir O (2012) Pigm Resin Technol 41:179
Patil RV, Ranganath MR, Lobo B (2011) International Conference on Condensed and Nano Materials 1393:371
Ramesan MT (2012) J Appli Polym Sci 128:1540
Mavinakuli P, Wei S, Wang Q, Karki AB, Dhage S, Wang Z, Young DP, Guo Z (2010) J Phys Chem 114:3874
Al-Saigh ZY (1999) Polym 40:3479
Haynes WM (ed) (2011) CRC Handbook of Chemistry and Physics, 91st edn. CRC Press Inc., Boca Raton
CAS Registry, the American Chemical Society, 1994-2012, Columbus, Ohio, USA. https://www.cas.org
Zou QC, Zhang SL, Wang SM, Wu LM (2006) J Chromatogr A 1129:255
Papadopoulou SK, Panayiotou C (2012) J Chromatog A 1229:230
Prolongo MG, Masegosa RM, Horta A (1989) Macromolecules 22:4346
Shi ZH, Schreiber HP (1991) Macromolecules 24:3522
Al-Saigh ZY, Chen P (1991) Macromolecules 24:3788
Jagiello J, Ligner G, Papirer E (1990) J Colloid Interface Sci 137:128–136
Hildebrand JH, Scott RL (1964) The Solubility of Non-Electrolytes. Dover Publishing, New York
Tarada M, Marchessault RH (1999) Int J Biol Macromol 25:207
Munk P (1993) IUPAC Int. Symp. Macromol. 34:185
Mangaraj D, Patra S, Rashid S (1963) Makromol Chem 65:39
Chee KK (1995) J. Applied Polym. Sci. 68:2057
Ramanaiah S, Rani PR, Sreekanth TVM, Reddy KS (2011) J Macromol Sci B 50:551
Barton AFM (1975) Chem Rev 75:731
Harris FW, Seymour RB (eds) (1977) Structure-solubility relationships in polymers. Academic Press, New York
Kozlowska MK, Domanska U, Rogalski MJ (2005) J Chromatogr A 1068:297
Guillet JE (1970) J Macromol Sci Chem 4:1669
DiPaola_Barayanyi G, Guillet JE (1978) Macromol 11:228
Fowkes FM (1964) Ind Eng Chem 561:40
Fowkes FM (1967) Ind Eng Chem Prod Res. Dev 56:40
Ramanaiah S, Rani PR, Sreekanth TVM, Reddy KS (2012) Polym Bull 50:551
Funding
This study was not funded by any agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author A, G. Ballard, declares that she has no conflict of interest. Author B, Z. Y. Al-Saigh, declares that he has no conflict of interest.
Ethical Approval
Involvement of Animals: This article is not involved with animals. Involvement of Human: This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
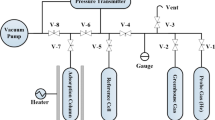
The key term in the IGC experiment is the specific retention volume Vog which enables the determination of thermodynamic parameters of a system under study, degree of crystallinity and the dispersive component of the surface energy. By measuring several main experimental chromatographic quantities such as; the flow rate, column temperature, retention time of test compounds, mass of the polymer, and the pressures of the carrier gas at the inlet and the outlet of the column, Vog can be calculated as follows:
J is the correction for the compressibility of the carrier gas across the chromatographic column defined by the following relation;
Here, Δt = tp − tm is the difference between the retention time of the test compound, tp and of the marker, tm. Air is usually used as a marker to account for the dead volume in the chromatographic column when the thermal conductivity (TC) detector is used. The retention time of the marker must be subtracted from the test compound retention time to reflect the test compound value of the test compound retention time as Δt. F is the flow rate of the carrier gas measured at the column temperature Tc, and w is the mass of the stationary phase. J factor is calculated using Pi and Po, the inlet and outlet pressures, respectively. Pi and Po are measured using electronic transducers, which are interfaced at the inlet and outlet of the column. These transducers are usually calibrated using a mercury manometer. Since Δt is a function of F, it must be extrapolated to zero to reflect the true value of Δt. Then, the product of the Δt and the flow rate F may also yield a valuable quantity as a net retention volume, VN, as follows:
VN in Eq. (6) accounts for the retention time of test compound in terms of volume in mL and it is dependent on the mass of the polymer in the column. To be more specific, VN can be taken a step further by dividing it by the mass of the polymer and corrected to 0 °C to become a specific retention volume of the test compound, Vog as in Eq. (4).
Vog is the key term for the calculations of thermodynamic quantities. It enables the calculations of the partition coefficient, Kp, which will lead to the calculation of the molar free energy of sorption (ΔGs) of the test compounds into the polymer layer, using the following relationship:
where ρ is the density of the polymer in the chromatographic column. Then, the relationship between Kp and the ΔGs is well known:
Accordingly, the molar heat of sorption (\(\Delta H_{s}^{1}\)) of test compounds into the polymer layer can also be derived from IGC data as follows [23]. For a pure solvent, the liquid–vapor equilibrium is described by the Clapeyron equation:
Here \(\bar{V}_{\text{g}}\) and \(\bar{V}_{\text{l}}\) are partial molar volumes of the test compound gas and liquid states and \(\Delta H_{s}^{1}\) is the partial molar heat of sorption of the test compound onto the polymeric surface. An analogous relationship can be derived for sorption of test compounds into the polymer layer (\(\Delta H_{s}^{1}\)):
Considering that \(\bar{V}_{l}\) is negligible as compared to \(\bar{V}_{\text{g}}\) and substituting the pressure of the vapor from the ideal gas equation for \(P_{1} = {\raise0.7ex\hbox{${\text{RT}}$} \!\mathord{\left/ {\vphantom {{\text{RT}} {\bar{V}_{\text{g}} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\bar{V}_{\text{g}} }$}}\)(this is always allowed when IGC set-up is at an infinite dilution). Using Vog from the chromatographic quantities along with Eq. (11), the molar heat of sorption of solvents into the polymer layer can be calculated as:
A plot of ln Vog versus the inverse of temperature will determine \(\Delta H_{s}^{1}\). If the polymer surface is amorphous at the experiments’ temperature, the equilibrium between the vapor and the polymer will be established, then the slope of the linear relationship [29] can be measured from the following equation.
To calculate the interaction parameters of test compounds used with PPY/TiO2, Vog from Eq. (4) can also be utilized to calculate the interaction parameter of the mobile and the stationary phases as, χ12, as follows:
χ12 parameter can reveal the strength of the specific interactions between the test compounds and the pure polymers and with the polymer pair. 1 denotes the test compound and 2 denotes the polymer under examination; υ2 is the specific volume of the polymer at the column temperature Tc; M1 is the molecular weight of the test compound; Po1 is the saturated vapor pressure of the test compound; V1 is the molar volume of the test compound; R is the gas constant; and B11 is the second virial coefficient of the test compound in the gaseous state. Equation (14) is used routinely for the calculation of χ12 from IGC experiments.Vog can also be utilized to calculate another important thermodynamic quantity, the molar heat of mixing of solvents with PPY/TiO2′s surface at infinite dilution, \( \Delta H_{1}^{\infty } \). To accomplish this goal, the mass fraction activity coefficient,\( \varOmega_{1}^{\infty } \), should be calculated [20].
The molar enthalpy of mixing \( \Delta H_{1}^{\infty } \) can be calculated using Eq. (15), as follows:
Similarly, the molar free energy and the molar entropy of mixing can also be calculated:
Solubility Parameters
To calculate the Hildebrand solubility parameters for the solvent, δ1 and the conducting PPY/TiO2, δ2 [44, 50, 51], the molar heat of vaporization of the solvent, ΔHv1 needs to be known. It is calculated using the following equation [52].
The solubility parameter of the solvent, δ1 is related to ΔHv1 by the following equation [44]
In the early 1970s, Guillet et al. [53, 54] were able to calculate solubility of the polymer, δ2, using the IGC method, they derived the following equation utilizing Flory–Huggins parameter, χ12, Eq. (14), as follows,
δ2 can be obtained from Eq. (21) by plotting the left-hand side of the equation versus δ1 calculated in Eq. (20), the slope of the straight line is (2 δ2/RT).
Dispersive Surface Energy
The total surface energy of PPY-TiO2 is the combination of several contributions depending on the nature of the solute that is interacting with the polymer surface. One of these contributions is due to the dispersive interaction forces such as those in alkanes. The dispersive forces in alkanes increase as more CH2 group is added to its backbone. If the solute used exhibits acid–base interaction forces, it also contributes to the total value of the surface energy. When the mobile gaseous phase comes in contact with the polymeric surface, an interfacial energy will be created according to the nature of the interacting solute and whether it is polar (γp) and/or non-polar or dispersive (γd). Then, the adsorption of the solute vapor onto the polymer surface will be affected by the magnitude of the surface free energy. Since the alkane series used in this work has a uniform increase in dispersive forces, it makes it possible to calculate the dispersive component of the surface energy of PPY/TiO2 using IGC. From the measured chromatographic quantities Vog can also be used to calculate the dispersive component of the surface energy of polymers using alkane test compounds [55, 56]. A complete theoretical treatment for the calculation of γds was first published by Fowkes [55]. It relates to the equilibrium constant K between the adsorbed test compound and the polymer surface, the molar free energy of adsorption, ∆Gs1, can be calculated as follows:
Equation (23) relates the energy of adsorption to the surface energy as follows:
γds and γdi are the dispersive components of the surface energy for the solid surface and the interactive test compounds, respectively. Equation (23) can be rewritten to yield the dispersive surface energy as follows:
where γCH2 is the surface energy of a hydrocarbon consisting only of n-alkanes, aCH2 is the area of one –CH2– group. Equation (24) usually tests the IGC method for obtaining the dispersive surface energy of polymers.
Rights and permissions
About this article
Cite this article
Ballard, G., Al-Saigh, Z.Y. Solubility and Surface Thermodynamics of Conducting Polymers by Inverse Gas Chromatography, IV: Polypyrrole/Titanium Oxide. Chromatographia 83, 451–466 (2020). https://doi.org/10.1007/s10337-019-03841-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03841-7