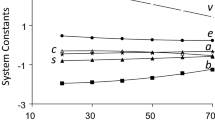
Abstract
The system constants of the solvation parameter model are used to prepare system maps for the retention of small neutral compounds on phenylhexylsiloxane- and pentafluorophenylpropylsiloxane-bonded superficially porous silica stationary phases (Kinetex Phenyl-Hexyl and Kinetex F5) for aqueous mobile phases containing 10–70% (v/v) methanol or acetonitrile. Electrostatic interactions (cation exchange) are important for the retention of weak bases for acetonitrile–water mobile phases, but virtually absent for the same compounds for methanol–water mobile phases. The selectivity of the Kinetex Phenyl-Hexyl stationary phase for small neutral compounds is similar to an octadecylsiloxane-bonded silica stationary phase with similar morphology Kinetex C-18 for both methanol–water and acetonitrile–water mobile phase compositions. The Kinetex Phenyl-Hexyl and XBridge Phenyl stationary phases with the same topology but different morphology are selectivity equivalent, confirming that solvation of the interphase region can be effective at dampening selectivity differences for modern stationary phases. Small selectivity differences observed for XTerra Phenyl (different morphology and topology) confirm previous reports that the length and type of space arm for phenylalkylsiloxane-bonded silica stationary phases can result in small changes in selectivity. The pentafluorophenylpropylsiloxane-bonded silica stationary phase (Kinetex F5) has similar separation properties to the phenylhexylsiloxane-bonded silica stationary phases, but is not selectivity equivalent. However, for method development purposes, the scope to vary separations from an octadecylsiloxane-bonded silica stationary phase (Kinetex C-18) to “phenyl phase” of the types studied here is limited for small neutral compounds. In addition, selectivity differences for the above stationary phases are enhanced by methanol–water and largely suppressed by acetonitrile–water mobile phases. For bases, larger selectivity differences are possible for the above stationary phases if electrostatic interactions are exploited, especially for acetonitrile-containing mobile phases.











Similar content being viewed by others
References
Snyder LR, Dolan JW, Marchand DA, Carr PW (2015) The hydrophobic subtraction model of reversed-phase column selectivity. Adv Chromatogr 50:297–376
Kayillo S, Dennis GR, Shalliker RA (2007) Retention of polycyclic aromatic hydrocarbons on propyl-phenyl stationary phases in reversed-phase high performance liquid chromatography. J Chromatogr A 1148:168–176
Begnini FR, Jardin ICSF (2013) Preparation and characterization of a new microwave immobilized poly(2-phenylpropyl)methylsiloxane stationary phase for reversed-phase high-performance liquid chromatography. J Chromatogr A 1297:113–122
Ashu-Arrah BA, Glennon JD, Albert K (2013) Synthesis, characterization and chromatographic evaluation of pentafluorophenyl and phenyl bonded silica phases prepared using supercritical fluid carbon dioxide as a reaction solvent. J Chromatogr A 1273:34–43
Bocian S, Buszewski B (2014) Phenyl-bonded stationary phases. The influence of polar functional groups on retention and selectivity in reversed-phase liquid chromatography. J Sep Sci 37:3435–3442
Yang M, Pezio S, Munch D, Drumm P (2005) Impact of methanol and acetonitrile on separations based on π–π interactions with a reversed-phase phenyl column. J Chromatogr A 1097:124–129
Janas P, Bocian S, Jandera Kowalkowski PT, Buszewski B (2016) Separation of flavonoids on different phenyl-bonded stationary phases—the influence of polar groups in stationary phase structure. J Chromatogr A 1429:198–206
Marchand DH, Croes K, Dolan JW, Snyder LR, Henry RA, Kallury KMR, Waite S, Carr PW (2005) Column selectivity in reversed-phase liquid chromatography—VIII phenylalkyl and fluoro-substituted columns. J Chromatogr A 1062:65–78
Croes K, Steffans AS, Marchand DH, Snyder LR (2005) Relevance of π–π and dipole–dipole interactions for retention on cyano and phenyl columns in reversed-phase liquid chromatography. J Chromatogr A 1098:123–130
Stevenson PG, Mayfield KJ, Soliven A, Dennis GR, Gritti F, Guiochon G, Shalliker RA (2010) π-Selective stationary phases: (I) influence of the spacer chain length of phenyl phases on the aromatic and methylene selectivity of aromatic compounds in reversed-phase high performance liquid chromatography. J Chromatogr A 1217:5358–5364
Euerby MR, Peterson P, Campbell W, Roe W (2007) Chromatographic classification and comparison of commercially available reversed-phase liquid chromatographic columns containing phenyl moieties using principal component analysis. J Chromatogr A 1154:138–151
Stevenson PG, Soliven A, Dennis GR, Gritti F, Guiochon G, Shalliker RA (2010) π-Selective stationary phases. (III) Influence of the phenyl ligand density on the aromatic and methylene selectivity of aromatic compounds in reversed-phase liquid chromatography. J Chromatogr A 1217:5377–5383
Stevenson PG, Gritti F, Guiochon G, Mayfield KJ, Dennis GR, Shalliker RA (2010) π-Selective stationary phases: (II) adsorption behavior of substituted aromatic compounds on n-alkyl-phenyl stationary phases. J Chromatogr A 1217:5365–5376
Bocian S, Skoczylas M, Gorynska I, Matyska M, Pesek J, Buszewski B (2016) Solvation process on phenyl-bonded stationary phases—the influence of polar functional groups. J Sep Sci 39:4369–4376
Bell DS, Jones AD (2005) Solute attributes and molecular interactions contributing to “U-shape” retention on a fluorinated high-performance liquid chromatography stationary phase. J Chromatogr A 1073:99–109
Bell DS, Cramer HM, Jones AD (2005) Rational method development strategies on a fluorinated liquid chromatography stationary phase: mobile phase concentration and temperature effects on the separation of ephedrine alkaloids. J Chromatogr A 1095:113–118
Gilar M, Yu Y-Q, Ahn J, Fournier J, Gebler JC (2008) Mixed mode chromatography for fractionation of peptides, phosphopeptides, and sialylated glycopeptides. J Chromatogr A 1191:162–170
Euerby MR, McKeown AP, Petersson P (2003) Chromatographic classification and comparison of commercially available perfluorinated stationary phases for reversed-phase liquid chromatography using principal component analysis. J Sep Sci 26:295–306
Grebenstein N, Frank J (2012) Rapid baseline-separation of all eight tocopherols and tocotrienols by reversed-phase liquid chromatography with solid-core pentafluorophenyl column and their sensitive quantification in plasma and liver. J Chromatogr A 1243:39–46
Verado V, Rieiputi Y, Garrido-Frenich A, Caboni MF (2015) Determination of free and bound phenolic compounds in soy isoflavone concentrate using a PFP fused-core column. Food Chem 185:239–244
Reta M, Carr PW, Sadek PC, Rutan SC (1999) Comparative study of hydrocarbon, fluorocarbon, and aromatic bonded RP-HPLC stationary phases by linear solvation energy relationships. Anal Chem 71:3484–3496
Neue UD, VanTran K, Iraneta PC, Alden BA (2003) Characterization of HPLC packings. J Sep Sci 26:174–186
Johnson AR, Johnson CM, Stoll DR, Vitha MF (2012) Identifying orthogonal and similar reversed-phase liquid chromatographic stationary phases using the system selectivity cube and the hydrophobic subtraction model. J Chromatogr A 1249:62–82
Poole CF, Lenca N (2017) Applications of the solvation parameter model in reversed-phase liquid chromatography. J Chromatogr A 1486:2–19
Poole CF, Poole SK (2002) Column selectivity from the perspective of the solvation parameter model. J Chromatogr A 965:263–299
Lesellier E, West C (2007) Description and comparison of chromatographic methods for packed column classification. J Chromatogr A 1158:329–360
Sykora D, Vozka J, Tesarova E (2016) Chromatographic methods enabling the characterization of stationary phases and retention prediction in high-performance liquid and supercritical fluid chromatography. J Sep Sci 39:115–131
Poole CF (2015) An interphase model for retention in liquid chromatography. J Planar Chromatogr 28:98–105
Chan FC, Yeung LS, LoBrutto R, Kazakevich YV (2005) Interpretation of the excess adsorption isotherms of organic eluent components on the surface of reversed-phase phenyl modified adsorbents. J Chromatogr A 1082:158–165
Vitha MF, Carr PW (2006) The chemical interpretation and practice of linear solvation energy relationships in chromatography. J Chromatogr A 1126:143–194
Abraham MH, Ibrahim A, Zissmos AM (2004) Determination of sets of solute descriptors from chromatographic measurements. J Chromatogr A 1037:29–47
Poole CF, Atapattu SN, Poole SK, Bell AN (2009) Determination of solute descriptors by chromatographic methods. Anal Chim Acta 652:32–53
Poole CF, Ariyasena TC, Lenca N (2013) Estimation of the environmental properties of compounds from chromatographic measurements and the solvation parameter model. J Chromatogr A 1317:85–104
Atapattu SN, Poole CF, Praseuth MB (2016) System maps for retention of small neutral compounds on a biphenylsiloxane-bonded silica stationary phase in reversed-phase liquid chromatography. J Chromatogr A 1478:68–74
Poole CF, Poole SK (2009) Foundations of retention in partition chromatography. J Chromatogr A 1216:1530–1550
Atapattu SN, Poole CF, Praseuth MB (2016) System maps for retention of small neutral compounds on a superficially porous particle column in reversed-phase liquid chromatography. J Chromatogr A 1468:250–256
Lenca N, Poole CF (2015) A system amp for the ionic liquid stationary phase 1,9-di(3-vinylimidazolium)nonane bis(trifluoromethylsulfonyl)imide. Chromatographia 78:81–88
Poole CF, Ahmed H, Kiridena W, DeKay C, Koziol WW (2005) Contribution of steric repulsion to retention on an octadecylsiloxane-bonded silica stationary phase in reversed-phase liquid chromatography. Chromatographia 62:553–561
Carr PW, Dolan JW, Neue UD, Snyder LR (2011) Contributions to reversed-phase column selectivity. 1. Steric interaction. J Chromatogr A 1218:1724–1742
Poole CF, Kiridena W, DeKay C, Koziol WW, Rosencrans RD (2006) Insights into the retention mechanism on an octadecylsiloxane-bonded silica stationary phase (HYPURITY C18) in reversed-phase liquid chromatography. J Chromatogr A 1115:133–141
McCalley DV (2010) The challenges of the analysis of basic compounds by high performance liquid chromatography: some possible approaches for improved separations. J Chromatogr A 1217:858–880
Bocian S, Buszewski B (2012) Residual silanols at reversed-phase silica in HPLC: contribution to a better understanding. J Sep Sci 35:91–1200
McDonald PD (2003) Improving our understanding of the reversed-phase separations for the 21st century. Adv Chromatogr 42:323–375
Kiridena W, Atapattu SN, Poole CF, Koziol WW (2008) Comparison of the separation characteristics of the organic–inorganic hybrid stationary phases XBridge C8 and Phenyl and XTerra Phenyl in RP-LC. Chromatographia 68:491–500
Atapattu SN, Poole CF, Praseuth MB (2017) System maps for the retention of small neutral compounds on a superficially porous ethyl-bridged octadecylsiloxane-bonded silica stationary phase in reversed-phase liquid chromatography. Chromatographia 80:1279–1286
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Atapattu and Poole have no conflict of interest. Author Praseuth is an employee of Phenomenex who manufactured the column used in this study. Authors Atapattu and Poole received no financial support from Phenomenex for this study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Atapattu, S.N., Poole, C.F. & Praseuth, M.B. Insights into the Retention Mechanism for Small Neutral Compounds on Silica-Based Phenyl Phases in Reversed-Phase Liquid Chromatography. Chromatographia 81, 225–238 (2018). https://doi.org/10.1007/s10337-017-3451-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3451-7