Abstract
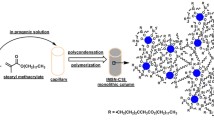
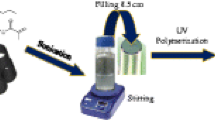
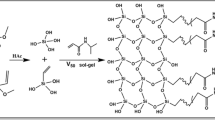
Analytical scale silica monoliths are commercially limited to three column selectivities (bare silica, C8 and C18). An in situ modification is reported in detail to overcome this barrier and allow for any functionality of choice to be bonded to the silica surface of the monolithic stationary phase support. The modification method was conducted on a commercial bare silica column to bond the C18 moiety to the silica surface through a silylation reaction. The C18 type of stationary phase was chosen, as this is the most commonly bonded functionality for the majority of stationary phases used for high-performance liquid chromatography (HPLC) separations. The C18-modified monolith’s performance was compared to a commercial C18 monolithic and a particle packed column of the same analytical scale column dimensions (100 × 4.6 mm). The modified C18 monolith proved to be of high quality with an efficiency of 73,267 N m−1, fast analysis times (operated at flow rates up to 3 mL min−1 using a conventional 400 bar HPLC system) and improved resolution of a set of polar and non-polar substituted aromatics in comparison to a commercial C18 monolith.









Similar content being viewed by others
References
Nakanishi K, Soga N (1991) Phase separation in gelling silica-organic polymer solution: systems containing poly(sodium styrenesulfonate). J Am Ceram Soc 74:2518–2530
Nakanishi K, Soga N (1992) Phase separation in silica sol–gel system containing polyacrylic acid. IV. Effect of chemical additives. J Non Cryst Solids 142:45–54
Nakanishi K, Soga N (1992) Phase separation in silica sol–gel system containing polyacrylic acid. III. Effect of catalytic condition. J Non Cryst Solids 142:36–44
Nakanishi K, Soga N (1992) Phase separation in silica sol–gel system containing polyacrylic acid. I. Gel formation behavior and effect of solvent composition. J Non Cryst Solids 139:1–13
Nakanishi K, Soga N (1992) Phase separation in silica sol–gel system containing polyacrylic acid. II. Effects of molecular weight and temperature. J Non Cryst Solids 139:14–24
Nakanishi K, Takahashi R, Soga N (1992) Dual-porosity silica gels by polymer-incorporated sol–gel process. J Non Cryst Solids 147:291–295
Nakanishi K, Soga N (1993) Inorganic porous column. Japan patent 5-200, 392
Nakanishi K, Soga N (1993) Production of inorganic porous body. Japan patent 5-208,642
Nakanishi K, Soga N (1997) Inorganic porous material and process for making same. US patent 5,624,875
Lubda D, Muller E (2003) Method for producing monolithic chromatography. US patent application 2003/0155676 a1
Siouffi AM (2003) Silica gel-based monoliths prepared by the sol–gel method: facts and figures. J Chromatogr A 1000:801–818
Qiu H, Liang X, Sun M, Jiang S (2011) Development of silica-based stationary phases for high-performance liquid chromatography. Anal Bioanal Chem 399:3307–3322
Guiochon G (2007) Monolithic columns in high-performance liquid chromatography. J Chromatogr A 1168:101–168
Cabrera K (2004) Applications of silica-based monolithic HPLC columns. J Sep Sci 27:843–852
Ali I, Gaitonde VD, Aboul-Enein HY (2009) Monolithic silica stationary phases in liquid chromatography. J Chromatogr Sci 47:432–442
Engelhardt H, Orth P (1987) Alkoxy silanes for the preparation of silica based stationary phases with bonded polar functional groups. J Liq Chromatogr 10:1999–2022
Buchmeiser MR (2001) New synthetic ways for the preparation of high-performance liquid chromatography supports. J Chromatogr A 918:233–236
Engelhardt H (2011) Bonded stationary phases. Chromatogr Sci 101:47–75
Hanai T (2000) New developments in liquid-chromatographic stationary phases. Adv Chromatogr 40:315–357
Silanes (2012) Gelest Incorporated, Morrisville, USA. http://www.gelest.com/gelest/forms/GeneralPages/prod_list.aspx?pltype=1&classtype=silanes. Accessed 15 Oct 2012
Soliven A, Dennis GR, Guiochon G, Hilder EF, Haddad PR, Shalliker RA (2010) Cyano bonded silica monolith—development of an in situ modification method for analytical scale columns. J Chromatogr A 1217:6085–6091
van Deemter JJ, Zuiderweg FJ, Klinkenberg A (1956) Longitudinal diffusion and resistance to mass transfer as causes of nonideality in chromatography. Chem Eng Sci 5:271–289
Miyabe K, Guiochon G (2003) Measurement of the parameters of the mass transfer kinetics in high performance liquid chromatography. J Sep Sci 26:155–173
Kirkland JJ, Snyder LR (1979) Introduction to modern liquid chromatography. Wiley, New York
Stevenson PG, Soliven A, Dennis GR, Gritti F, Guiochon G, Shalliker RA (2010) Pi-selective stationary phases: (III) Influence of the propyl phenyl ligand density on the aromatic and methylene selectivity of aromatic compounds in reversed phase liquid chromatography. J Chromatogr A 1217:5377–5383
Stevenson PG, Soliven A, Dennis GR, Shalliker RA (2009) Phenyl-type and C1 stationary phases for environmentally friendlier chromatography. J Sep Sci 32:3880–3889
Capello C, Hellweg S, Badertscher B, Betschart H, Hungerbühler K (2007) Environmental assessment of waste-solvent treatment options. Part I. The ecosolvent tool. J Ind Ecol 11:26–38
Snyder LR, Dolan JW (2007) High-performance gradient elution the application of the linear-solvent-strength model. Wiley, Hoboken
Gritti F, Guiochon G (2011) Measurement of the eddy diffusion term in chromatographic columns I. Application to the first generation of 4.6 mm I.D monolithic columns. J Chromatogr A 1218:5216–5227
Acknowledgments
This work was supported by the Australian Research Council’s Discovery funding scheme (DP0987318). E.F.H. is the recipient of an ARC Future Fellowship (FT0990521). We gratefully acknowledge Dr. Thomas Rodemann (Central Science Laboratory, University of Tasmania) for assistance with elemental analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soliven, A., Dennis, G.R., Hilder, E.F. et al. The Development of the In Situ Modification of 1st Generation Analytical Scale Silica Monoliths. Chromatographia 77, 663–671 (2014). https://doi.org/10.1007/s10337-014-2667-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2667-z