Abstract
Competition for nectar is expected to result in feeding niche differentiation. Here, we targeted the sexually size-dimorphic Volcano Sunbird (Cinnyris preussi) on Mount Cameroon. We investigated whether males and females feed on different plant species, whether females with shorter bills than males prefer shorter flowers, and whether larger dominant males visit more energetic flowers that produce higher amounts of nectar sugars. We also asked whether feeding niches were spatially separated along the elevation gradient and whether this separation varied between the two contrasting seasons. We collected data on the frequency of visits to individual plant species and analyzed the male-to-female ratios in the mist-netted dataset. In addition, we estimated production of nectar sugar in individual habitats and seasons. Despite the large dataset collected, encompassing 6476 bird–plant interactions, our findings did not provide evidence of differences in the spectra of the visited plant species. In addition, females did not visit flowers with shorter tubes, nor did males visit flowers that produced higher amounts of sugars. However, we observed a sex-specific dispersion of sunbirds during the wet season. During the dry breeding season, both males and females feed mainly in nectar-rich montane and submontane forests. In the wet season, the production of nectar sugar in these habitats decreased dramatically, and females largely disappeared. In contrast, female activity increased in the lowest and highest parts of the altitudinal range. Our findings on elevational movements are important in the current context, in which species face potential threats from habitat destruction and climate change.
Zusammenfassung
Aufteilung von Nahrungsressourcen zwischen Männchen und Weibchen eines Nektarvogels (Cinnyris preussi) auf dem Kamerunberg.
Es wird erwartet, dass die Konkurrenz um Nektar zu einer Differenzierung in der Nahrungsnische führt. Wir untersuchten hier einen Nektarvogelart (Cinnyris preussi) auf dem Kamerunberg, die einen Geschlechtsdimorphismus aufweist. Wir prüften, (1) ob Männchen und Weibchen sich von verschiedenen Pflanzenarten ernähren, (2) ob Weibchen, die einen kürzeren Schnabel als Männchen haben, Blüten mit kürzeren Blütenkelchen bevorzugen und (3) ob die größeren und dominanten Männchen energiereichere Blüten aufsuchen, die größere Mengen an Nektarzucker produzieren. Wir wollten auch wissen, ob die Nahrungsnischen entlang des Höhengradienten räumlich getrennt sind und ob diese räumliche Trennung zwischen den beiden gegenteiligen Jahreszeiten variiert. Wir sammelten Daten über die Besuchshäufigkeit an einzelnen Pflanzenarten und analysierten das Männchen-Weibchen-Verhältnis von in den Japannetzen gefangenen Individuen. Darüber hinaus schätzten wir die Produktion von Nektarzucker in den verschiedenen Lebensräumen und Jahreszeiten. Trotz des großen Datensatzes, der 6.476 Vogel-Pflanzen-Interaktionen umfasste, erbrachten unsere Ergebnisse keine Hinweise auf Unterschiede im Spektrum der aufgesuchten Pflanzenarten. Darüber hinaus besuchten weder die Weibchen gezielt Blumen mit kürzeren Blütenkelchen auf, noch besuchten Männchen Blüten, welche die größeren Mengen an Zucker produzierten. Jedoch beobachteten wir eine geschlechtsspezifische Verteilung der Nektarvögel während der Regenzeit. Während der trockenen Brutzeit gingen sowohl die Männchen als auch die Weibchen vor allem in nektarreichen montanen und submontanen Wäldern auf Nahrungssuche. In der Regenzeit ging die Produktion von Nektarzucker in diesen Lebensräumen drastisch zurück, und die Weibchen verschwanden größtenteils. Im Gegenzug dazu nahm die Aktivität der Weibchen in den niedrigsten und höchsten Teilen des Höhengebiets zu. Unsere Ergebnisse zu den Höhenbewegungen sind wichtig im aktuellen Kontext, in dem Arten durch Zerstörung ihrer Lebensräume und Klimawandel bedroht sind.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competition among nectar-feeding birds is a well-known phenomenon. It can have a character of either simultaneous exploitation of nectar from the same flowers or of a direct interference for resources among individuals (Ford 1979). Regardless of its nature, competition incurs associated costs, and nectarivorous birds tend to mitigate it through niche differentiation (Gill 1978; Janeček et al. 2012). This can be achieved by niche segregation in space, time, or feeding on different co-flowering plant species. Spatial segregation can occur at different scales. Birds can feed on different parts of the same plant (Stiles and Wolf 1970; Ford and Paton 1982; Lara et al. 2009), separate their feeding territories in the same habitat (Lara et al. 2009), use different habitats in relatively small areas (Ford and Paton 1976; Ortiz-Pulido et al. 2012), or migrate to distant, less competitive environments (Bertin 1980; McKinney et al. 2012). Similarly, temporal segregation can occur over short periods, for instance birds can visit the same plants in a given area at different times of the day (Lara et al. 2009) or in different seasons (Araújo and Sazima 2003; Araújo et al. 2013). Differences in the spectra of visited plant species are often related to trait matching between bill and floral morphologies (Kodric-Brown et al. 1984; Janeček et al. 2012; Maglianesi et al. 2014; Weinstein and Graham 2017; Sonne et al. 2019). The competition and need for niche differentiation are not the same throughout the year, but can intensify in times of nectar food shortage (Yeaton and Laughrin 1976; Tinoco et al. 2017). During nectar-poor periods, some habitats can become profitable only for some bird species, whereas other birds need to move to more profitable areas (Wolf 1970; Ford 1979; Collins 1985).
Moreover, feeding niche differentiation can be a consequence of both interspecific and intraspecific competitive interactions, with sexual differentiation serving as a special example of the latter. On a small spatial scale, niche differentiation between the sexes is often related to territorial behavior. Colwell and King (unpublished, cited in Feinsinger and Colwell 1978) observed that males of Green-backed Firecrown (Sephanoides sephaniodes) were strongly territorial, whereas females were low-reward trapliners or generalists. Similarly, male Olive Sunbirds (Cyanomitra olivacea) defend nectar-rich sources (Frost and Frost 1981). Nevertheless, not only males but also females of hummingbirds (Wolf 1969) or sunbirds (Gill and Wolf 1975) can be territorial.
Sex-specific migration of nectarivorous birds is known to occur in hummingbirds. The males of Broad-tailed Hummingbirds (Selasphorus platycercus) disappear from the breeding locality when the feeding conditions become unfavorable, while females stay to take care of the young (Wagner 1945). In addition, sex-specific migrations of the Mexican Violetear (Colibri thalassinus) have been reported. While adult females, young males, and some adult males migrate, other adult males stay during the winter in the neighborhood of the breeding range, feeding on remnants of flowering plants (Wagner 1945). In Mato Grosso do Sul, Brazil, males of Black-throated Mango (Anthracothorax nigricollis) were observed feeding on plants for only one month, whereas females were also recorded in other periods (Barbosa-Filho and Araujo 2013). In addition, males of Allen's Hummingbird (Selasphorus sasin) precede the migration of females from breeding sites (Phillips 1975). Although various movements of other specialized nectarivores are known (e.g., Keast 1968; Craig and Hulley 1994), to the best of our knowledge, information on possible sex differences is missing.
When both sexes occupy the same space, the segregation of feeding niches can be based on differences in their food plant spectra. This segregation is often associated with sexual size dimorphism in birds (Temeles et al. 2010; Berns and Adams 2013; Maglianesi et al. 2022). Sexual size dimorphism has been observed in many phylogenetic lineages of nectarivorous birds. In the Old World, it is relatively rare in honeyeaters (Clarke and Clarke 1999) but common in sunbirds (Cheke et al. 2001). In the New World, sexual size dimorphism is widespread among hummingbirds (Berns and Adams 2013). Males are larger than females in larger-bodied hummingbird species, whereas the opposite is true for smaller species (Colwell 2000; Avalos et al. 2022). It was shown that the long-billed females of Purple-throated Carib (Eulampis jugularis) have significantly shorter handling times on deep flowers of the green morph of Heliconia bihai than the short-billed males (Temeles et al. 2009). Similarly, the long-billed females of Mountain Velvetbreast (Lafresnaya lafresnayi) feed on a different spectrum of plants than males (Snow and Snow 1980), and the females of Fork-tailed Woodnymph (Thalurania furcata) feed on floral resources, which exhibit more similarity to another hummingbird species Planalto Hermit (Phaethornis pretrei) than T. furcata males (Faria and Araújo 2010). Feeding on different plants can also be related to different energy needs, which are a consequence of dimorphism in body size (Brown et al. 1978) or behavior (Riegert et al. 2011).
In our study, we targeted the niche differentiation of the sexually size-dimorphic Volcano Sunbird (Cinnyris preussi) on Mount Cameroon. We explored its feeding behavior, spatial distribution, and changes in these characteristics in two contrasting seasons (wet and dry). Our study was performed in five vegetation types along an elevational gradient, and we also investigated the differences in nectar production. We tested several hypotheses and related predictions that originated mainly from hummingbird studies: (1) The sexes of nectarivorous birds can segregate their feeding niches to reduce intraspecific competition. This can be achieved by feeding in the same area on different plants or by spatial movement and feeding in different areas. We expected C. preussi females and males to forage on different plant species. During the breeding (dry) season, we anticipate that both sexes will occupy the same geographical area; however, in the non-breeding (wet) season, they will exhibit varying altitudinal distribution patterns. (2) The distinction in niches becomes particularly pronounced during periods of limited food availability. We expected lower nectar sugar production and, consequently, higher niche segregation during the wet season. (3) Plant–pollinator interactions are shaped by mutual trait matching. We expected that C. preussi males with longer bills would be more likely to visit long tubular flowers. (4) Body mass is positively related to energy demand and social dominance. We expect that larger males will be dominant over females and, in consequence, will chase the females from the plants; in the case of movement, the females will move to areas with a lower amount of resources. Finally, we expected heavier and socially dominant C. preussi males to visit flowers with higher sugar production.
Methods
Study site
Mt. Cameroon is the highest mountain in West and Central Africa and is a global biodiversity hotspot (Myers et al. 2000; Küper et al. 2004; Hoffman et al. 2016). A diverse range of tropical forests can be observed on the slopes of Mt. Cameroon. Despite the extensive destruction and transformation of lowland littoral forests on the southwestern foothills into plantations, pristine forests can be encountered at elevations of approximately 400 m above the sea level. In some areas, these forests have experienced natural disturbances due to the presence of local African Forest Elephant (Loxodonta cyclotis) populations (Maicher et al. 2020a). The timberline location varies at different sites, typically falling within the range of 1900–2500 m above sea level. Above the timberline, a wide spectrum of herbaceous communities can be observed ascending to the summit, which is approximately 4040 m above sea level (electronic supplementary material Fig. S1 and Fig. S2). Mt. Cameroon experiences conspicuous seasonality. During the wet season, monthly precipitation locally exceeds 2000 mm, whereas almost no rain occurs during the dry season (Maicher et al. 2020b).
We performed our study at five locations along the altitudinal gradient (electronic supplementary material Fig. S2 and Table S1): (1) the mid-elevational forest (MEF) around the PlanteCam Camp (1100 m a. s. l.), which is partially disturbed by elephants; (2) the submontane forest mosaic (SF) around the Crater Lake locality (1500 m a.s.l.), which is characterized by large elephant pastures and patches of forest; (3) the montane forest (MF) around Mann’s Spring (2100 m a. s. l.), near the timberline; (4) the low-elevational montane grasslands (LG) above the timberline near Hut 1 on the Guinness Trail (2100 m a. s. l.), and (5) the high-elevational montane grasslands (HG) near Hut 2 (2800 m a. s. l.). Sampling was performed for approximately 8 weeks at each elevation. Four weeks during the wet season and 4 weeks during the dry season.
Studied species
Volcano Sunbird (Reichenow 1892, Fig. S1) inhabits the highlands of Cameroon, Equatorial Guinea (Bioko) and Nigeria. This taxon was often classified as a subspecies of the Northern Double-collared Sunbird (C. reichenowi) (Cheke et al. 2001) under which name it has appeared in the vast majority of sunbird–plant interaction studies (e.g., Janečková et al. 2021; Sejfová et al. 2021; and other cited studies). Nevertheless, it was recently shown to be distinct from that of East African species (Cooper et al. 2021).
C. preussi has, similar to other double-collared sunbirds, pronounced sexual dimorphism. Females are inconspicuously colored, while males display conspicuous coloring. Females are smaller (t-test, t-value = 11.12, p < 0.0001; average female mass: 8.23 ± 0.06 g (SE), n = 167; average male mass: 9.14 ± 0.05 g (SE), n = 261, our unpublished data; electronic supplementary material Fig. S3) with shorter culmen length, that is the distance from the base of the feather on the culmen to the tip measured as a straight line (t-test, t-value = 7.89, p < 0.001; average female culmen length: 16.40 ± 0.13 mm (SE), n = 65; average male culmen length: 17.77 ± 0.10 mm (SE), n = 140; data from Cooper et al. 2021; electronic supplementary material Fig. S3).
C. preussii feeds on insects and on nectar (Cheke et al. 2001; Riegert et al. 2011). C. preussii visits flowers with a broad spectrum of floral morphologies. It drinks not only from open, morphologically generalized, but also from tubular, morphologically specialized flowers (Janeček et al. 2007, 2011, 2012, 2022). The females lay eggs during the transition from the wet to dry season and the dry season (Serle 1951; Cheke et al. 2001, our personal observations).
Plant trait data collection
To examine the degree of trait matching between C. preussi and the flowers they visit, we collected morphological and nectar data of the flowers. We measured tube length using an electronic caliper. We measured at least five randomly selected flowers per species, each from a different plant individual. To estimate nectar sugar production over a 24-h period, we initially marked randomly selected opened flowers on the target plant individual and covered them with mesh bags to prevent nectar consumption by floral visitors. After 24 h, we used these flowers for nectar sampling. Each sample was represented by flowers from one plant individual. We collected at least three samples per species for the rarest plants, but usually at least 15 samples for the common species. To estimate the production of nectar sugar, two nectar-processing methods were used, based on nectar production (1) from flowers with high nectar production, we extracted nectar and measured nectar volume using microcapillary tubes or Hamilton syringes. We measured the nectar concentration using a Pal-1 (Atago Co.) pocket refractometer. (2) When nectar production was too low, we washed the flower with filtered water, transferred it with diluted sugars into an Eppendorf tube, added ethanol, and boiled it for 15 min to deactivate the enzymes (Chlumská et al. 2014). In the laboratory, samples were dried and transferred to constant volumes. The concentrations of individual sugars (i.e., glucose, fructose, and sucrose) were measured by high-performance liquid chromatography using an ICS-3000 system (Dionex) with an electrochemical detector and a CarboPac PA 1 column. The sugar amount (mg) per flower was calculated from both nectar-processing methods. For other information on nectar production see Bartoš et al. (2012) and Janeček et al. (2021) and for floral traits measurements Klomberg et al. (2022). For the analyses, we used the mean tube length and mean nectar sugar production per flower averaged across individual plants within each species.
Transects and estimation of plant community nectar sugar production
To estimate plant abundance and nectar sugar production per unit area, we established six transects for each vegetation type. Each transect was 0.2 ha large, 200 m long, and 10 m wide. In each transect, we counted the number of plants and flowers visited by C. preussi during the wet and dry seasons. For nectar sugar production assessment per area, the number of flowers was multiplied by species-specific 24-h nectar sugar production per flower.
Bird observation
The data on plant–C. preussi interactions were partly extracted from the larger datasets on plant–nectivorous bird interactions in Mt. Cameroon forest (Janeček et al. 2022) and partly from unpublished datasets from Mt. Cameroon montane grasslands. In this study, only the plants visited by C. preussi were considered. We observed birds using timed observations of plant species occurring in or around transects. Compared to transect walking, this method provides sufficient sampling effort for relatively rare and/or rarely visited plants (Gibson et al. 2011), which can nevertheless be a highly specialized bird-pollinated species (Janeček et al. 2015). We designed the observation of plants to be as effective as possible, depending on the environment and plant size. In the tropical forests, we monitored the herbs and smaller shrubs using security cameras (Vivotek IB8367) with a target to record ten individuals of each plant species and each plant individual for two days. Trees and shrubs, which did not fit the camera field view, were observed personally with the target to observe eight individuals of each plant species each for eight hours (for more details, see Janeček et al. 2022). In montane grasslands, where the vegetation is much more open, we observed more plants at one observation point. In grasslands, we also recorded the chasing behavior of males and females. We aimed to observe at least ten individuals of each plant species at different observation points. The average observation time for each plant species was 150 h. Nevertheless, the rarity of some species, together with various logistical and/or technical problems related to the harsh weather on Mt. Cameroon, resulted in different recording times for individual plant species (electronic supplementary material Table S2). Young birds of indeterminate sex, that is, female-like but with a specific behavior and/or bill yellow base, were not considered in this study. Nevertheless, during the study period, the number of these birds was relatively low (4.3% of mist-netted birds were young for which we were not able to determine the sex).
Bird trapping
To support the plant visitation data on C. preussi female-to-male ratios in individual vegetation types and seasons, we report bird-trapping data. These data were obtained from two large datasets collected in two projects. The first project was conducted along the forest elevational gradient (partly used by Kamga et al. 2023), whereas the second focused on the grasslands above the timberline. In all locations, we used 16 mm mesh size nets to maximize the chances of catching small passerines such as C. preussi. In MEFs and MFs, birds have been caught using ground-to-canopy mist nests (Chmel et al. 2016, 2021; Kamga et al. 2023) and a few ordinary ground nets. At other sites, 10–15 ground nets (12 m each) were used. The nets were operated at the same time for three consecutive days. However, due to the difficulties imposed by the terrain, the length of the nets used varies across locations. When the weather conditions allowed, the nets were opened from 06:00 AM to 06:00 PM.
Statistical analyses
To compare the observed numbers of males and females with the predicted numbers in the ratio of 1:1, we used the chi-square test. The associations between sex and season were tested in 2 × 2 contingency tables using Fisher’s exact tests. Statistical analyses were conducted using R software (R Core Team 2023).
Visitation frequencies per hour were calculated first per plant and then per area. To estimate the frequency of visits per area, we used 0.2 ha transects. The frequency of visits per transect (T) was estimated for each transect as:
where Ai is the number of individuals of plant species i on the transect and Fi is the mean frequency of visits per one individual of plant species i. The data on visitation frequencies by males and females on individual plants contained many zeros, and consequently, were not normally distributed. Therefore, we tested the effects of the study site, season, bird sex, and their interactions using the nonparametric permutation test in the PERMANOVA program, which is an extension of the PRIMER software (Anderson et al. 2008). Plant ID was nested as a random factor in study site × season. Visitation frequency data were log (x + 1) transformed to decrease the effect of extreme values.
The effects of season and study site on biotope nectar sugar production were tested by repeated-measures ANOVA in the program Statistica, with factor season considered as a repeated-measures factor (TIBCO Software Inc. 2020).
We calculated the food niche overlap using a bird sex versus plant species matrix. The cell entries represent the interaction frequencies (i.e., the number of visits per hour). Niche overlap was expressed as Morista’s similarity index (Morisita 1959) using the niche overlap function in the R package spaa (Zhang 2016). The index ranges from 0 (the sexes do not share any plants) to 1 (they feed with the same frequency on the same resources). We tested the observed overlap with the null modeled overlaps, which were calculated after 1000 randomizations of visitation matrix data. In each randomization we (1) created the C. preussi sex vs. plant individuals matrix with the cell entries as the number of visits (2) which were then randomly redistributed with the restrictions that the row and column totals were fixed. In other words, the total number of visits by individual bird sex and the total number of visits to individual plants did not change. (3) The bird sex × plant individuals matrix was recalculated into a bird sex × plant species matrix with cells being the interaction frequencies, and the null model Morista’s similarity index was calculated.
We tested the relationship between the proportion of female visits on individual plant species (dependent variable) and their mean tube length and nectar sugar production per flower using linear regressions in the Statistica program (TIBCO Software Inc. 2020). Although proportions, the data distribution on female visits did not significantly differ from the normal distribution (Kolmogorov–Smirnov test, d = 0.14, p > 0.20) and consequently we did not use any data transformation.
Results
In total, we recorded 6476 visits by C. preussi on 44 plant species (Fig. 1), including 4137 males and 2339 females. The total number of visits by males and females significantly differed from the 1:1 ratio (Chi-square = 499.2, p < 0.001). There was also a significant association between bird sex and season (Fisher’s exact test, p < 0.001). The male-to-female ratio of observed visits was lower in the dry season (1.32:1) and higher in the wet season (3.38:1). The differences between sexes in the number of observed visits were significant in both the dry (Chi-square = 83.27, p < 0.001) and wet (Chi-square = 653.76, p < 0.001) seasons. The number of visits by males and females also differed from the 1:1 ratio in the individual study sites and seasons, except for the highest elevation in the dry season (Table 1). When significant, more visits by males were always observed, except for the lowest elevation in the MEF during the wet season, with a much higher number of observed females (Fig. 1; Table 1). The season-bird sex associations differed in three of the four tested study sites (Table 1). At all these sites, there was a higher proportion of females observed during the dry season. No C. preussi individuals were observed in the MEF during the dry season; consequently, this test was not performed. The study site, season, bird sex, and their interactions significantly influenced the frequency of visits, regardless of whether they were calculated per plant or per area (Table 2). The visitation frequency patterns of males and females were similar in the dry season, but differed in the wet season. In the dry season, both males and females had the highest visitation frequencies in the MFs and SFs when calculated per plant and area, respectively. In the wet season, the frequency per plant and per area increased in montane grasslands, but decreased in SFs. During the wet season, females had the highest frequencies per plant and per unit area at both the highest and the lowest elevations (Fig. 2).
Feeding of C. preussi males and females on flowering plants in different vegetation types and seasons. The sizes of the bars, which represent the plant species, are proportional to the visitation frequency per plant. VEGETATION TYPES: MEF—mid-elevation forest; SF—submontane forest; MF—montane forest; LG—the low-elevational montane grasslands; HG—the high-elevational montane grasslands. PLANTS: Aca dec—Acanthopale decempedalis; Afr sp.—Aframomum sp.; Ant sca—Anthocleista scandens; Ast aby—Astropanax abyssinicum; Bri owa—Brillantaisia owariensis; Cla ani—Clausena anisata; Cle sim—Clematis simensis; Cle sil—Clerodendrum silvanum; Cli rob—Clinopodium robustum; Cos dub—Costus dubius; Dic ves—Dicranolepis vestita; Eng gab—Englerina gabonensis; Hyp rev—Hypericum revolutum; Hyp tri—Hypoestes triflora; Imp bur—Impatiens burtonii; Imp eti—Impatiens etindensis; Imp fri—Impatiens frithii; Imp hia—Impatiens hians; Imp nia—Impatiens niamniamensis; Imp sak—Impatiens sakeriana; Ixo fol—Ixora foliosa; Ixo gui—Ixora guineensis; Jas pre—Jasminum preussii; Kig afr—Kigelia africana; Las gla—Lasiosiphon glaucus; Leu oli—Leucas oligocephala; Lob col—Lobelia collumnaris; Mim sol—Mimulopsis solmsii; Mus ten—Mussaenda tenuiflora; Nux con—Nuxia congesta; Phy sch—Phyllopentas schimperi; Ple dec—Plectranthus decurrens; Ple kam—Plectranthus kamerunensis; Psy ped—Psychotria peduncularis var. hypsophila; Psy dun—Psydrax dunlapii; Rha den—Rhabdotosperma densifolia; Rhi sp.—Rhipidoglossum sp.; Sol pse—Solanum pseudospinosum; Suc tri—Succisa trichotocephala; Syz sta—Syzygium staudtii; Syz sp.—Syzygium sp.; Tab ven—Tabernaemontana ventricosa; Tho san—Thonningia sanguinea; Thu fas—Thunbergia fasciculata
Visitation frequencies of males and females of C. preussi in different study sites and seasons are expressed as the number of visits per area (upper part) or per plant (lower part). HG—the high-elevational montane grasslands; LG—the low-elevational montane grasslands; MF—the montane forest; SF—the submontane forest mosaic; MEF—the mid-elevational forest. Box: mean ± SE; Whisker: mean ± SD
In total, we mist-netted 513 C. preussi individuals: 289 males and 224 females. The total number of mist-netted males and females significantly differed from the 1:1 ratio (Chi-square = 8.24, p = 0.004). There was a significant association between sex and season (Fisher’s exact test, p < 0.001). The male-to-female catching ratio was 0.93:1 in the dry season, and the difference between the number of caught males and females was not significant (Chi-square = 0.37, p = 0.543), whereas it was 1.89:1 in the wet season, and the difference was highly significant (Chi-square = 23.15, p < 0.001). The male-to-female ratios did not differ from the 1:1 ratio at the individual sites during the dry season (Table 3). In the wet season, there were significantly more males in the HG and MF. The number of males was also higher (but not significantly) at other sites, except for the lowest study site in the MEF (Table 3).
Nectar sugar production at individual study sites along the elevational gradient differed between seasons (repeated-measures ANOVA, Study site: F = 3.55, p = 0.020; Season: F = 9.21, p = 0.006, Season*Study site: F = 3.14, p = 0.032). At all study sites, higher nectar sugar production was recorded in the dry season, and when all study sites were combined, nectar sugar production was eight times higher than that in the wet season. The highest sugar production by plants visited by C. preussi was recorded in the MF, followed by the SF, in the dry season (Fig. 3). In this time, the nectar sugar production was driven by flowering trees such as Astropanax abyssinicum (Araliaceae), Nuxia congesta (Stilbaceae) in MF, or Tabernaemontana ventricosa (Apocynaceae) in SF. During the wet season, the highest nectar sugar source was Psychotria peduncularis var. hypsophila (Rubiaceae) in MFs, and various Impatiens spp. (Balsaminaceae), Anthocleista scandens (Gentianaceae) or Clerodendrum silvanum (Lamiaceae) in the lower elevations. In both montane grassland sites, nectar sugar production was driven by Hypericum revolutum (Hypericaceae) in the dry season and by Phyllopentas schimperi (Rubiaceae), and partly by H. revolutum in the wet season.
We did not find any evidence of male versus female niche segregation in the spectrum of the visited plant species because the observed niche overlaps did not differ from the overlaps generated by the null models (Table 4). This was true not only for individual site-season combinations but also for specific models for individual seasons and the entire model, including visitation frequencies for all studied plants.
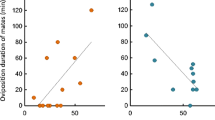
When comparing the traits of plants visited by C. preussi and the proportion of visits by females, we did not find any significant effect of nectar sugar production (r = −0.0293, p = 0.854) or tube length (r = 0.14, p = 0.357). Similarly, insignificant differences were observed in separate analyses for the dry (nectar sugar production: r = −0.03, p = 0.873; tube length r = 0.13; p = 0.526) and wet (nectar sugar production: r = −0.08, p = 0.698; tube length r = 0.33, p = 0.100) seasons. In addition, the unimodal distribution of floral lengths of the visited plant species did not indicate niche partitioning (electronic supplementary material Fig. S4).
On montane grasslands, where we have been recording chasing behavior, we observed a significantly higher number of attacks by males than by females (males: n = 63, females: n = 1; chi-square = 60.06, p < 0.001). Males attacked other males more often (n = 39) than females (n = 24). Nevertheless, this difference was only marginally significant (chi-square = 3.57, p = 0.059). The only recorded attack by a female was on another female. No attacks by females on males were recorded.
Discussion
Despite the huge dataset on C. preussi–plant interactions that we collected, we did not confirm our expectation that males and females would segregate their feeding niches by visiting different plant spectra when staying at the same locality. The highest number of bird visits by both sexes was recorded in MF and SF during the dry season when the availability of nectar sugar was high. Dominant males have been observed chasing females from plants. In times of food shortage, when C. preussi do not breed, females have partly disappeared from the MF and SF, and were much more frequently observed in the upper and lower parts of the altitudinal range.
We suppose that the absence of expected trait matching, evident in the similar spectra of plants visited by males and females as well as the lack of correlation between the proportion of female visits and both floral tube length and nectar sugar production, can be explained by several factors. The most important factor seems to be the high proportion of nectar-rich generalized plants that are frequently visited by sunbirds. This was true mainly in the dry season, when the observed niche overlap was notably high, although not significantly greater than that predicted by the null model. In the MF, during the dry season, trees Astropanax abyssinicum, Nuxia congesta, and Syzigium staudtii were the plants most frequently visited by C. preussi (Fig. 1; Janeček et al. 2022). In the SF, during the dry season, the most visited plants were trees Syzigium sp. and Tabernaemontana ventricosa. The flowers of these trees do not have floral tubes (A. abyssinicum, Syzigium spp.), or the tubes are relatively short (N. congesta); consequently, nectar can be easily accessed by both males and females. T. ventricosa has a bit longer floral tube, but it is a plant adapted to moths and in consequence does not fit the morphology of sunbirds' bills. In montane grasslands, one of the most visited plants in both the dry and wet seasons is Hypericum revolutum, a bee-pollinated plant with flat, morphologically generalized flowers (Fig. S1a; Janeček et al. 2007; Bartoš et al. 2015). Specialized ornithophilous plants that specialize in bird pollination predominantly flower on Mt. Cameroon during the wet season (Janeček et al. 2011, 2015; Bartoš and Janeček 2014; Klomberg et al. 2022). In addition, Uceda-Gómez et al. (2024) demonstrated on sunbird species level that trait matching is more important during this season. Nevertheless, precise trait matching in the entire sunbird-plant network seems to be obscured by asymmetry in the relationship between ornithophilous plants and sunbirds (Chmel et al. 2021). Sunbirds do not care much and visit many other non-specialized plants in both the wet and dry seasons (Chmel et al. 2021; Janeček et al. 2022). Moreover, the absence of expected segregation could, to some extent, be caused by the relatively small differences in male and female bill lengths. Although less effective, nectar can be harvested by a protruding tongue and may be taken from flowers deeper than the bill length (Collins 2008). Unfortunately, information on sex specificity or similarity in plant spectra for other sunbird species is absent or fragmentary (e.g., Hobbhahn and Johnson 2015). Our results do not support the results of studies on hummingbirds, which showed that size-dimorphic nectarivorous birds feed on different plant spectra (Taylor and White 2007; Faria and Araújo 2010). Nevertheless, this could also be the result of more pronounced sexual size dimorphism and higher specialization in some hummingbird species (Colwell 2000; Avalos et al. 2022).
In contrast to the differences in floral spectra, our results indicate spatial niche separation. On the small scale, we often observed the chasing of subordinate females by dominant males. This is in agreement with other studies on sunbird territoriality and male dominance (Frost and Frost 1981; Evans and Hatchwell 1992). During other studies in Bamenda Highlands (Cameroon), near our field station we even observed a male of C. preussi chasing out his own female from some plants of Hypoestes aristata but letting her drink on others (see also Padyšáková et al. 2013). In the same locality, C. preussi has been observed to protect H. aristata from other pollinating species, including carpenter bees (Tropek et al. 2013). Nevertheless, as demonstrated for Rufous Hummingbirds (Selasphorus rufus), this space and resource division does not necessarily need to be disadvantageous for females, and they can compensate for it with lower costs and greater success in robbing from rich male territories (Carpenter et al. 1993). Our results are in accordance with previous observations that C. preussi individuals move along the altitude during the wet season (Serle 1951; Cheke et al. 2001). Nevertheless, this movement is not only a simple shift from the MF to lower elevations, as expected (Serle 1951; Cheke et al. 2001), but also a more complex and sex-specific dispersion from the MF upward and downwards. However, ringing and/or bird tracking data are required to obtain a more accurate picture of these movements. Unfortunately, the use of both methods is so far not realistic, as the first method is limited by a low ringing effort on Mt. Cameroon, and the second by the absence of sufficiently light tracking devices to be carried by small sunbirds.
When comparing the male-to-female ratios in the observation and mist-netting datasets, two common trends were found: (1) a higher proportion of males in the montane and SF in the wet season compared to the dry season and (2) higher proportions of females in lowest elevation during the wet season. The most obvious difference was the much higher proportion of males in the flower visitation dataset than in the mist-netting dataset during the dry season. We assume that this can be caused by higher territoriality in the dry season associated with breeding and/or a high abundance of C. preussi in the montane and SFs. Males visit plants not only because they are hungry but also as part of the territory-defending strategy when they aim to keep the levels of nectar in the flowers as low as possible (Paton and Carpenter 1984). Nevertheless, mist netting can also be biased to some extent by the different behaviors of the individual sexes (Borgella et al. 2001).
To gain comprehensive knowledge of male and female movement, it is necessary to explore other slopes and/or habitats. For example, we observed that during wet seasons, both C. preussi females and males fed on garden plants in the village of Bokwango (suburban area of Buea, approximately 900 m a. s. l.), but they disappeared in the dry season. Similarly, the limitation of our study is that sampling in each vegetation type and season was performed only once, that is, during one expedition. Consequently, we have not been able to reveal the potential temporal variability across years. Although this study aimed to test more general hypotheses, it also has an important message for conservation. The local migration of sunbirds is not well known, and only a few anecdotal observations have been reported for some species (Cheke et al. 2001). Nevertheless, these local migrations and movements face several conservation challenges, such as long-distance migrations (Bairlein 2016; Flack et al. 2022). For example, long-distance migrants and locally moving birds are dependent on resources in several habitats. Consequently, these birds can also be, compared to the sedentary species, more threatened by habitat destruction, habitat loss or climatic changes. In the case of nectarivores, climatic changes can influence the synchronization of habitat flowering. Consequently, the presence of nectivorous birds and convenient plant flowering may be mismatched (McKinney et al. 2012). However, nectarivorous birds can find new resources in anthropogenic habitats, such as urban areas, gardens, and flower plantations (Maruyama et al. 2019; Janeček et al. 2020). These threats require further studies to be incorporated into conservation planning.
Data availability
The datasets are available from the corresponding author upon reasonable request.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Araújo AC, Sazima M (2003) The assemblage of flowers visited by hummingbirds in the “capões” of southern Pantanal, Mato Grosso do Sul, Brazil. Flora 198:427–435. https://doi.org/10.1078/0367-2530-00116
Araújo FP, Sazima M, Oliveira PE (2013) The assembly of plants used as nectar sources by hummingbirds in a Cerrado area of Central Brazil. Plant Syst Evol 299:1119–1133. https://doi.org/10.1007/s00606-013-0783-0
Avalos G, Triana F, Klank J (2022) Variation in sexual size dimorphism and fit to Rensch’ s rule in 45 species of Costa Rican hummingbirds. Res Square. https://doi.org/10.21203/rs.3.rs-1992118/v1
Bairlein F (2016) Migratory birds under threat. Science 354:547–548. https://doi.org/10.1126/science.aah6647
Barbosa-Filho WG, de Araujo AC (2013) Flowers visited by hummingbirds in an urban Cerrado fragment, Mato Grosso do Sul, Brazil. Biota Neotrop 13:21–27. https://doi.org/10.1590/S1676-06032013000400001
Bartoš M, Janeček Š (2014) Pollinator-induced twisting of flowers sidesteps floral architecture constraints. Curr Biol 24:793–795. https://doi.org/10.1016/j.cub.2014.07.056
Bartoš M, Janeček Š, Padyšáková E, Patáčová E, Altman J, Pešata M, Kantorová J, Tropek R (2012) Nectar properties of the sunbird-pollinated plant Impatiens sakeriana: a comparison with six other co-flowering species. S Afr J Bot 78:63–74. https://doi.org/10.1016/j.sajb.2011.05.015
Bartoš M, Tropek R, Spitzer L, Padyšáková E, Janšta P, Straka J, Tkoč M, Janeček Š (2015) Specializaton of pollination systems of two co-flowering phenotypically generalized Hypericum species (Hypericaceae) in Cameroon. Arthropod Plant Interact 9:241–252. https://doi.org/10.1007/s11829-015-9378-8
Berns CM, Adams DC (2013) Becoming different but staying alike: patterns of sexual size and shape dimorphism in bills of hummingbirds. Evol Biol 40:246–260. https://doi.org/10.1007/s11692-012-9206-3
Bertin RI (1980) The Ruby-throated Hummingbird and its major food plants: ranges, flowering phenology, and migration. Can J Zool 60:210–219. https://doi.org/10.1139/z82-029
Borgella R, Snow AA, Gavin TA (2001) Species richness and pollen loads of hummingbirds using forest fragments in southern Costa Rica. Biotropica 33:90–109. https://doi.org/10.1111/j.1744-7429.2001.tb00160.x
Brown JH, Calder WA III, Kodric-Brown A (1978) Correlates and consequences of body size in nectar-feeding birds. Am Zool 18:687–700. https://doi.org/10.1093/icb/18.4.687
Carpenter FL, Hixon MA, Temeles EJ, Russell RW, Paton DC (1993) Exploitative compensation by subordinate age-sex classes of migrant Rufous Hummingbirds. Behav Ecol Sociobiol 33:305–312. https://doi.org/10.1007/BF00172928
Cheke RA, Mann CF, Allen R (2001) Sunbirds: a guide to the sunbirds, flowerpeckers, spiderhunters and sugarbirds of the world. Yale University Press, New Haven
Chlumská Z, Janeček Š, Doležal J (2014) How to preserve plant samples for carbohydrate analysis? Test of suitable methods applicable in remote areas. Folia Geobot 49:1–15. https://doi.org/10.1007/s12224-013-9153-5
Chmel K, Riegert J, Paul L, Novotný V (2016) Vertical stratification of an avian community in New Guinean tropical rainforest. Popul Ecol 58:535–547. https://doi.org/10.1007/s10144-016-0561-2
Chmel K, Kamga SM, Awa T, Ewome FL, Uceda-Gómez G, Hořák D, Mlíkovský J, Molua LL, Riegert J, Janeček Š (2021) Vertical stratification and seasonal changes of the avian community in Mount Cameroon lowland rainforest. Afr J Ecol 59:655–666. https://doi.org/10.1111/aje.12877
Clarke RH, Clarke MF (1999) The social organization of a sexually dimorphic honeyeater: the Crescent Honeyeater Phylidonyris pyrrhoptera, at Wilsons Promontory, Victoria. Aust J Ecol 24:644–654. https://doi.org/10.1046/j.1442-9993.1999.00990.x
Collins BG (1985) Energetic of foraging and resource selection by honeyeaters in forest and woodland habitats of Western Australia. N Z J Zool 12:577–587. https://doi.org/10.1080/03014223.1985.10428307
Collins BG (2008) Nectar intake and foraging efficiency: responses of honeyeaters and hummingbirds to variations in floral environments. Auk 125:574–587. https://doi.org/10.1525/auk.2008.07070
Colwell RK (2000) Rensch’s rule crosses the line: convergent allometry of sexual size dimorphism in hummingbirds and flower mites. Am Nat 156:495–510. https://doi.org/10.1086/303406
Cooper JC, Maddox JD, McKague K, Bates JM (2021) Multiple lines of evidence indicate ongoing allopatric and parapatric diversification in an Afromontane sunbird (Cinnyris reichenowi). Ornithology 138:1–21. https://doi.org/10.1093/ornithology/ukaa081
Craig JFK, Hulley PE (1994) Sunbird movements: a review, with possible models. Ostrich 65:106–110. https://doi.org/10.1080/00306525.1994.9639672
Evans MR, Hatchwell BJ (1992) An experimental study of male adornment in the Scarlet-tufted Malachite Sunbird: I. The role of pectoral tufts in territorial defense. Behav Ecol Sociobiol 29:413–419. https://doi.org/10.1007/BF00170171
Faria RR, Araújo AC (2010) Flowering phenology and pollination of ornithophilous species in two habitats of Serra da Bodoquena, Mato Grosso do Sul, Brazil. An Acad Bras Cienc 82:843–855
Feinsinger P, Colwell RK (1978) Community organization among neotropical nectar-feeding birds. Am Zool 18:779–795. https://doi.org/10.1093/icb/18.4.779
Flack A, Aikens EO, Kölzsch A, Nourani E, Snell KRS, Fiedler W, Linek N, Bauer HG, Thorup K, Partecke J, Wikelski M, Williams HJ (2022) New frontiers in bird migration research. Curr Biol 20:R1187–R1199. https://doi.org/10.1016/j.cub.2022.08.028
Ford HA (1979) Interspecific competition in Australian honeyeaters—depletion of common resources. Aust J Ecol 4:145–164. https://doi.org/10.1111/j.1442-9993.1979.tb01205.x
Ford HA, Paton DC (1976) Resource partitioning and competition in honeyeaters of genus Meliphaga. Aust J Ecol 1:281–287. https://doi.org/10.1111/j.1442-9993.1976.tb01118.x
Ford HA, Paton DC (1982) Partitioning of nectar sources in an Australian honeyeater community. Aust J Ecol 7:149–159. https://doi.org/10.1111/j.1442-9993.1982.tb01588.x
Frost SK, Frost PGH (1981) Sunbird pollination of Strelitzia nicolai. Oecologia 49:379–384. https://doi.org/10.1007/BF00347603
Gibson RH, Knott B, Eberlein T, Memmott J (2011) Sampling method influences the structure of plant–pollinator networks. Oikos 120:822–831. https://doi.org/10.1111/j.1600-0706.2010.18927.x
Gill FB (1978) Proximate costs of competition for nectar. Am Zool 18:753–768. https://doi.org/10.1093/icb/18.4.753
Gill FB, Wolf LL (1975) Economics of feeding territoriality in the golden-winged sunbird. Ecology 56:333–345. https://doi.org/10.2307/1934964
Hobbhahn N, Johnson SD (2015) Sunbird pollination of the dioecious root parasite Cytinus sanguineus (Cytinaceae). S Afr J Bot 99:138–143. https://doi.org/10.1016/j.sajb.2015.04.003
Hoffman M, Koenig K, Bunting G, Costanza J, Williams KJ (2016) Biodiversity hotspots (version 2016.1) (2016.1). Zenodo. https://doi.org/10.5281/zenodo.3261807
Janeček Š, Hrázský Z, Bartoš M, Brom J, Reif J, Hořák D, Bystřická D, Riegert J, Sedláček O, Pešata M (2007) Importance of big pollinators for the reproduction of two Hypericum species in Cameroon, West Africa. Afr J Ecol 45:607–613. https://doi.org/10.1111/j.1365-2028.2007.00779.x
Janeček Š, Patáčová E, Bartoš M, Padyšáková E, Spitzer L, Tropek R (2011) Hovering sunbirds in the Old World: occasional behaviour or evolutionary trend? Oikos 120:178–183. https://doi.org/10.1111/j.1600-0706.2010.18612.x
Janeček Š, Riegert J, Bartoš M, Hořák D, Reif J, Padyšáková E, Fainová D, Antczak M, Pešata M, Mikeš V, Patáčová E, Altman J, Kantorová J, Hrázský Z, Bróm J, Doležal J (2012) Food selection by avian floral visitors: an important aspect of plant–flower interactions in West Africa. Biol J Linn Soc 107:355–367. https://doi.org/10.1111/j.1095-8312.2012.01943.x
Janeček Š, Bartoš M, Njabo KY (2015) Convergent evolution of sunbird pollination systems of Impatiens species in tropical Africa and hummingbird systems of the new world. Biol J Linn Soc 115:127–133. https://doi.org/10.1111/bij.12475
Janeček Š, Chmel K, Uceda Gomez G, Janečkova P, Chmelová E, Sejfová Z, Luma Ewome F (2020) Ecological fitting is a sufficient driver of tight interactions between sunbirds and ornithophilous plants. Ecol Evol 10:1784–1793. https://doi.org/10.1002/ece3.5942
Janeček Š, Chmel K, Ewome FL, Hrubá K, Klomberg Y, Kobe IN, Kouede RD, Mertens JEJ, Njie MM, Tropek R (2021) Differences in nectar traits between ornithophilous and entomophilous plants on Mount Cameroon. Plants 10:1161. https://doi.org/10.3390/plants10061161
Janeček Š, Chmel K, Mlíkovský J, Uceda-Gómez G, Janečková P, Fominka NT, Njie MM, Ewome FL (2022) Spatiotemporal pattern of specialization of sunbird-plant networks on Mt. Cameroon. Oecologia 199:885–896. https://doi.org/10.1007/s00442-022-05234-4
Janečková P, Sejfová Z, Janeček Š, Chmel K, Chmelová E, Ewome FL (2021) Behavioural correlates of drinking speed in two West African sunbird species. Bird Stud 67:540–542. https://doi.org/10.1080/00063657.2021.1917510
Kamga SM, Tamungang SA, Awa T II, Chmel K, Luma Ewome F, Lyonga Molua L, Uceda-Gómez G, Janeček Š, Mlíkovský J, Riegert J (2023) Changes in bird community structure on Mount Cameroon driven by elevational and vertical gradients. Diversity 15:727. https://doi.org/10.3390/d15060727
Keast A (1968) Seasonal movements in the Australian honeyeaters (Meliphagidae) and their ecological significance. Emu 67:159–209. https://doi.org/10.1071/MU967159
Klomberg Y, Tropek R, Mertens JEJ, Kobe IN, Hodeček J, Raška J, Fominka NT, Souto-Vilarós D, Janečková P, Janeček Š (2022) Spatiotemporal variation in the role of floral traits in shaping tropical plant–pollinator interactions. Ecol Lett 25:839–850. https://doi.org/10.1111/ele.13958
Kodric-Brown A, Brown JH, Byers GS, Gori DF (1984) Organization of a tropical island community of hummingbird and flowers. Ecology 65:1358–1368. https://doi.org/10.2307/1939116
Küper W, Sommer JH, Lovett JC, Beentje HJ, van Rompaey RSAR, Chatelain C, Sosef M, Barthlott W (2004) Africa’s hotspots of biodiversity redefined. Ann Mo Bot Gard 91:525–535
Lara C, Lumbreras K, González M (2009) Niche partitioning among hummingbirds foraging on Penstemon roseus (Plantaginaceae) in central Mexico. Ornitol Neotrop 20:73–83
Maglianesi MA, Blüthgen N, Böhning-Gaese K, Schleuning M (2014) Morphological traits determine specialization and resource use in plant-hummingbird networks in the tropics. Ecology 95:3325–3334. https://doi.org/10.1890/13-2261.1
Maglianesi MA, Maruyama PK, Temeles EJ, Schleuning M, Zanata TB, Sazima M, Gutiérrez-Zamora A, Marín-Gómez OH, Rosero-Lasprilla L, Ramírez-Burbano MB, Ruffini AE, Salamanca-Reyes JR, Sazima I, Nuñez-Rosas LE, Arizmendi MC, Rahbek C, Dalsgaard B (2022) Behavioural and morphological traits influence sex-specific floral resource use by hummingbirds across the Americas. J Anim Ecol 91:2171–2180. https://doi.org/10.1111/1365-2656.13746
Maicher V, Delabye S, Murkwe M, Doležal J, Altman J, Kobe IN, Desmist J, Fokam EB, Pyrcz T, Tropek R (2020a) Effects of disturbances by forest elephants on diversity of trees and insects in tropical rainforests on Mount Cameroon. Sci Rep 10:21618. https://doi.org/10.1038/s41598-020-78659-7
Maicher V, Sáfián S, Murkwe M, Delabye S, Przybyłowicz Ł, Potocký P, Kobe IN, Janeček Š, Mertens JEJ, Fokam EB, Pyrcz T, Doležal J, Altman J, Hořák D, Fiedler K, Tropek R (2020b) Seasonal shifts of biodiversity patterns and species’ elevation ranges of butterflies and moths along a complete rainforest elevational gradient on Mount Cameroon. J Biogeogr 47:342–354. https://doi.org/10.1111/jbi.13740
Maruyama PK, Bonizário C, Marcon AP, D’Angelo G, da Silva MM, da Silva Neto EN, Oliveira PE, Sazima I, Sazima M, Vizentin-Bugoni J, dos Anjos L, Rui AM, Marçal Júnior O (2019) Plant–hummingbird interaction networks in urban areas: generalization and the importance of trees with specialized flowers as a nectar resource for pollinator conservation. Biol Conserv 230:187–194. https://doi.org/10.1016/j.biocon.2018.12.012
McKinney AM, CaraDonna PJ, Inouye DW, Barr B, Bertelsen CD, Waser NM (2012) Asynchronous changes in phenology of migrating broad-tailed hummingbirds and their early-season nectar resources. Ecology 93:1987–1993. https://doi.org/10.1890/12-0255.1
Morisita M (1959) Measuring of interspecific association and similarity between communities. Mem Fac Sci Kyushu Univ Ser E Biol 3:65–80. https://doi.org/10.18960/seitai.11.6_252_4
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Ortiz-Pulido R, Díaz SA, Valle-Díaz OI, Fisher AD (2012) Hummingbirds and the plants they visit in the Tehuacán-Cuicaltán biosphere reserve. Mexico Rev Mex Biodivers 83:152–163. https://doi.org/10.22201/ib.20078706e.2012.1.1139
Padyšáková E, Bartoš M, Tropek R, Janeček Š (2013) Generalization versus specialization in pollination systems: visitors, thieves, and pollinators of Hypoestes aristata (Acanthaceae). PLoS One 8:e59299. https://doi.org/10.1371/journal.pone.0059299
Paton DC, Carpenter FL (1984) Peripheral foraging by territorial Rufous hummingbirds: defense by exploitation. Ecology 65:1808–1819. https://doi.org/10.2307/1937777
Phillips AR (1975) The migration of Allen’ s and other hummingbirds. Condor 77:196–205. https://doi.org/10.2307/1365790
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reichenow A (1892) Zur Vogelfauna Von Kamerun. J Ornithol 40:177–195. https://doi.org/10.1007/BF02208272
Riegert J, Fainová D, Antzak M, Sedláček O, Hořák D, Reif J, Pešata M (2011) Food niche differentiation in two syntopic species: a case study from the Cameroon mountains. J Ornithol 152:819–825. https://doi.org/10.1007/s10336-011-0650-0
Sejfová Z, Mlíkovský J, Ewome FL, Janečková P, Klomberg Y, Njie MM, Janeček Š (2021) Sunbirds’ tendency to hover: The roles of energetic rewards, inflorescence architecture and rain. J Avian Biol 52:jav.02818. https://doi.org/10.1111/jav.02818
Serle W (1951) The double-collared sunbird Cinnyris reichenowi sharpe. Nigerian Field 16:20–21
Snow DW, Snow BK (1980) Relationship between hummingbirds and flowers in the Andes of Colombia. Bull Br Mus Nat Hist 38:105–139
Sonne J, Zanata TB, González AMM, Torres NLC, Fjeldså J, Colwell RK, Tinoco BA, Rahbek C, Dalsgaard B (2019) The distributions of morphologically specialized hummingbirds coincide with floral trait matching across an Andean elevational gradient. Biotropica 51:205–2018. https://doi.org/10.1111/btp.12637
Stiles FG, Wolf LL (1970) Hummingbird territoriality at a tropical flowering tree. Auk 87:467–491. https://doi.org/10.2307/4083791
Taylor J, White SA (2007) Observations of hummingbird feeding behaviour at flowers of Heliconia beckneri and H. tortuosa in southern Costa Rica. Ornitol Neotrop 18:133–138
Temeles EJ, Koulouris CR, Sander SE, Kress WJ (2009) Effect of flower shape and size on foraging performance and trade-offs in a tropical hummingbird. Ecology 90:1147–1160. https://doi.org/10.1890/08-0695.1
Temeles EJ, Miller JS, Rifkin JL (2010) Evolution of sexual dimorphism in bill size and shape of Hermit Hummingbirds (Phaethornithinae): a role for ecological causation. Phil Trans R Soc B 365:1053–1063. https://doi.org/10.1098/rstb.2009.0284
TIBCO Software Inc. (2020) Data science workbench, version 14. http://tibco.com
Tinoco BA, Graham CH, Aguliar JM, Schleuning M (2017) Effects of hummingbird morphology on specialization in pollination networks vary with resource availability. Oikos 126:52–60. https://doi.org/10.1111/oik.02998
Tropek R, Bartoš M, Padyšáková E, Janeček Š (2013) Interference competition between sunbirds and carpenter bees for the nectar of Hypoestes aristata. Afr Zool 48:392–394. https://doi.org/10.3377/004.048.0222
Uceda-Gómez G, Chmel K, Janečková P, Mlíkovský J, Klomberg Y, Ewome FL, Molua LL, Njie MM, Tropek R, Janeček Š (2024) Drivers of sunbird-plant interactions on Mount Cameroon: between neutrality and niche-based processes. Biotropica 56:136–148. https://doi.org/10.1111/btp.13290
Wagner HO (1945) Notes on the life history of the Mexican Violet-ear. Wilson Bull 57:165–187
Weinstein BG, Graham CH (2017) Persistent bill and corolla matching despite shifting temporal resources in tropical hummingbird-plant interactions. Ecol Lett 20:326–335. https://doi.org/10.1111/ele.12730
Wolf LL (1969) Female territoriality in a tropical hummingbird. Auk 86:490–504. https://doi.org/10.2307/4083410
Wolf LL (1970) The impact of seasonal flowering on the biology of some tropical hummingbirds. Condor 72:1–14
Yeaton RI, Laughrin L (1976) Fall resource division in Santa Cruz Island hummingbirds. Wilson Bull 88:272–279
Zhang J (2016) Package ‘spaa’. Version 0.2.2, Species association analysis. Retrieved from https://cran.r-project.org/web/packages/spa/spa.pdf
Acknowledgements
We want to thank Kryštof Chmel, Eko Evaristus Mwende, Kum Peter Abieja, Ndombo Rodulf Mokake, Ngotto John, Marcus Ngotto, Njie Motombi Francis, Lyonga Samuel, Klaus Basongo Elive, Collins Njie, Jakques Esembe, Mesango Robinson Matute and Joseph Ekema and many students for help in the field. We also thank Eric Djomo Nana and Nganje Jean Evakise for their help with logistics. We are grateful to everyone in the Bokwango community for their long-term support and hospitality.
Funding
Open access publishing supported by the National Technical Library in Prague. This research was supported by the Grant Agency Charles University (GAUK No. 383521) and the Czech Science Foundation (20-16499S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by C. T. Downs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janeček, Š., Uceda-Gómez, G., Janečková, P. et al. Food resource partitioning between males and females of Volcano Sunbird (Cinnyris preussi) on Mount Cameroon. J Ornithol (2024). https://doi.org/10.1007/s10336-024-02187-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10336-024-02187-8