Abstract
Many aspects of the spatial ecology of the cinereous vulture (Aegypius monachus) are still unknown. The Iberian population is thought to be predominantly sedentary, but the spatial patterns of young individuals have been barely studied. The aim of this study was to provide a comprehensive understanding of the juvenile dispersal of the Iberian cinereous vultures. To this end, we GPS-tagged 41 Cinereous Vultures and tracked their movements during the period 2002–2021, from the end of parental care to the beginning of reproductive age. We examined the influence of age, season and sex on movement metrics (accumulated distances, distances to nest and home range sizes). During the juvenile dispersal period, cinereous vultures travelled over large areas of the Iberian Peninsula and southern Europe. Despite the high individual variability, we found a negative age-related trend in all movement metrics: the younger individuals (<1 year old) often performed farther movements and occupied larger areas, stabilising their movements during immature (1–3 years) and subadult (4 years) phases. On the other hand, season influenced the accumulated distance within all age classes; warm months positively influenced flight effort. Finally, females flew farther and occupied larger areas than males, consistently within age classes. This study did not take into account many factors which may explain part of the high variability observed: landscape, supplementary feeding sites, dumps, colony size, interspecific interactions, stochastic events, etc. Further studies are needed to investigate the influence of these factors on the dispersal of the species in more detail, but this work provides the first approach to the juvenile dispersal of the cinereous vulture in Iberia.
Zusammenfassung
Alter, Jahreszeit und Geschlecht beeinflussen die Dispersion von Jungvögeln bei spanischen Mönchsgeiern (Aegypius monachus).
Viele Aspekte der räumlichen Ökologie des Mönchsgeiers (Aegypius monachus) sind noch unbekannt. Es wird angenommen, dass die iberische Population überwiegend sesshaft ist, aber die räumlichen Muster der Jungtiere sind kaum untersucht worden. Ziel dieser Studie war es, ein umfassendes Verständnis der Ausbreitung von Jungtieren der iberischen Mönchsgeier zu erlangen. Zu diesem Zweck haben wir 41 Mönchsgeier mit GPS-Tags versehen und ihre Bewegungen im Zeitraum 2002–2021 3 verfolgt, vom Ende der elterlichen Fürsorge bis zum Beginn des reproduktiven Alters. Wir untersuchten den Einfluss des Alters, der Jahreszeit und des Geschlechts auf die Bewegungsdaten (akkumulierte Entfernungen, Entfernungen zum Nest und Größe des Heimatgebiets). Während der Ausbreitungsperiode der Jungvögel überflogen Mönchsgeier große Gebiete der Iberischen Halbinsel und Südeuropas. Trotz der hohen individuellen Variabilität fanden wir einen negativen altersabhängigen Trend bei allen Bewegungsmaßen: Die jüngeren Individuen (<1 Jahr alt) führten häufig weitere Bewegungen durch und besetzten größere Gebiete, wobei sich ihre Bewegungen während der immaturen (1–3 Jahre) und subadulten (4 Jahre) Phase stabilisierten. Andererseits beeinflusste die Jahreszeit die kumulierte Entfernung in allen Altersklassen; die warmen Monate wirkten sich positiv auf die Flugleistung aus. Schließlich flogen die Weibchen weiter und besetzten größere Gebiete als die Männchen, und zwar durchweg innerhalb der Altersklassen. In dieser Studie wurden viele Faktoren nicht berücksichtigt, die einen Teil der beobachteten hohen Variabilität erklären könnten: Landschaft, zusätzliche Futterstellen, Mülldeponien, Koloniegröße, interspezifische Interaktionen, stochastische Ereignisse usw. Weitere Studien sind erforderlich, um den Einfluss dieser Faktoren auf die Ausbreitung der Art genauer zu untersuchen, aber diese Arbeit liefert den ersten Ansatz, die Dispersion von Jungvögeln des Mönchsgeiers auf der Iberischen Halbinsel zu verstehen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile dispersal of birds includes the period from the departure from the natal nest to the settlement at the first breeding site. This process involves wandering movements of young birds for months or years after fledging, often performing tens or hundreds of kilometres away from the natal nest, in all directions, without distinct seasonality (Greenwood and Harvey 1982, Newton 2010). Thus, birds acquire knowledge of the landscape, find suitable breeding areas, and develop flying and foraging skills. This strategy is widely adopted by young birds born within sedentary populations (Newton 2010). The success of birds during non-reproductive periods is essential for stabilising population sizes and ranges (Maness and Anderson 2013). However, due to the wandering and exploratory nature of these movements, juvenile dispersal is one of the stages with the highest mortality rates (Oppel et al. 2015; García-Macía et al. 2022a). The better knowledge of spatial–temporal patterns of juvenile dispersal, the better management tools to be implemented for the conservation of the species.
Age and seasonality are two of the most important factors that influence juvenile dispersal in raptors. First, as birds gain experience, they become more efficient and perform more regular movements, often reducing their wandering behaviour (Ferrer 1993; Balbotín Ferrer 2009; García-Macía et al 2022a). Second, seasonality brings about major changes on all levels: temperature and rainfall, day length, presence/absence of migratory species, adult breeding, etc. Therefore, strong shifts in environmental conditions, especially in template areas such as the Mediterranean region, may affect the spatial ecology of birds. These two combined factors, age and seasonality, should be studied during juvenile dispersal to understand the changes in the movements of young birds.
Sex may also influence the behaviour of some young raptors, although great variability within species and populations has been reported. Some female juvenile raptors travel further than males (Forsman et al. 2002; Whitfield et al. 2009), but sex is not considered to have a significant effect on many other species (Newton et al. 1989; Walls and Kenward 1995; Balbontín and Ferrer 2009; García-Macía et al. 2022a). It is expected that sex has a stronger effect on the spatial behaviour of adult birds because breeding events often entail role specialization (Martínez et al. 2020; López-López et al. 2021; García-Macía et al. 2022b). However, sexual size dimorphism (females bigger than males) also appears in juvenile raptors and leads to numerous differences in hunting behaviour (Krüger 2005; Panter and Amar 2022). Within monomorphic species, such as vultures, behavioural differences and spatial segregation between sexes might also appear (Morant et al. 2023), so the influence of sex in juvenile dispersal should be studied.
The cinereous vulture (Aegypius monachus), an obligate scavenger, is the biggest raptor in Europe (Cramps and Simmons 1980). Its range is irregularly extended from Iberia to Asia, including 8400–11,400 breeding pairs worldwide (BirdLife International 2021), and more than 2500 in Spain (Del Moral 2017). The current distribution area in Spain is the result of the recent increase in regional populations and re-introduction programs (Del Moral 2017). In the Iberian Peninsula, the cinereous vulture usually settles in Mediterranean forests or semi-open woodland formations (Carrete and Donázar 2005; Muñoz-Adalia and Hernández 2013). This species often feeds on small and medium-sized carrion, including lagomorphs, deer, wild boars and livestock (Hiraldo 1976, Costillo et al. 2007). Inter-specific interactions between Iberian cinereous vultures and other Iberian avian scavengers, such as griffon Vultures (Gyps fulvus), Egyptian vultures (Neophron percnopterus), kites (Milvus sp.) and corvids (Del Moral and De la Puente 2017) are frequent.
Many aspects of the spatial ecology of the species are still unknown. In the Caucasian region and Asia some individuals migrate (Kim et al. 2007; Gavashelishvili et al. 2012; Yamaç and Bilgin 2012; Kang et al. 2019), but it is considered mostly as resident in Iberia. In Spain, many studies on the species’ breeding biology (Hiraldo 1983; Moreno-Opo et al. 2010), foraging activity (Costillo et al. 2007), habitat selection (Carrete and Donázar 2005) or anthropic impacts (Morán-López et al. 2006; Hernández Margalida 2008; Iglesias-Merchán et al. 2016) have been conducted, but studies focused on the spatial ecology with high-precision remote tracking are necessary to understand how the species use the territory during the entire lifecycle.
Here, we GPS-tagged 41 Cinereous Vultures from the Iberian population to study the influence of sex, season and age in the wandering movements of young non-breeding individuals. Previous studies have reported the influence of those intrinsic and extrinsic factors in the juvenile dispersal of similar Iberian raptors (García-Macía et al. 2022a; Morant et al. 2023), being fundamental to understand their large-scale movements. Therefore, due to the juvenile dispersal of the Iberian cinereous vulture has been barely studied, it is fundamental to analyse how those factors determine their wandering movements in order to further understand the spatial behaviour of the species and have tools for its conservation. Thus, our specific objectives were: 1) To test sex differences in the juvenile dispersal movements, being expected that both sexes have similar behaviour because the influence of breeding is not present in young individuals. 2) To study differences between age classes (juveniles, immatures and subadults) in the dispersal movements of the species; a progressive stabilisation is expected from the end of parental care to adulthood. And 3) to explore seasonal differences in the juvenile dispersal movements, being expected that home range sizes and accumulated distances increase during spring and summer.
Materials and method
Tagging and sample size
Forty young cinereous vultures (18 males, 16 females, 6 with undetermined sex) were GPS-tagged at different colonies in Spain in the period 2002–2021. Individuals provided data for 2.5 ± 1.7 years (mean ± SD), in the range 1–5 years (ESM Table S1).
Thirty-four vultures were tagged as chicks in the nest when they reached a similar size to the adults, but were unable to fly. Six individuals were released as immatures as part of Spanish re-introduction projects. All individuals were ringed, weighed and measured, and a blood sample was taken for molecular sexing (except for six individuals; Ellegren 1996). A GPS-biologger transmitter was attached to the back of each individual by a back-pack harness tied with Teflon ribbon, designed to allow its release after a few years of monitoring.
Different transmitter models were attached to the individuals: Microwave PTT-100 70 g Solar Argos/GPS MTI (Microwave Telemetry Inc., Columbia, Maryland, USA; n = 28), OrniTrack-50 solar-powered GPS-GSM tracker (Ornitela, Vilnius, Lithuania; n = 9), E-obs Solar 48g GPS-GSM-GPRS (E-Obs GMBH, Gruenwald, Germany; n = 3), and Ecotone Saker-L (Ecotone Telemetry, Gdynia, Poland; n = 1). The weight of all transmitters was less than 5% of the birds’ weight, thus complying with the recommended standard (Kenward 2001).
Biologgers provided GPS fixes every 5 min to 2 h from dawn to dusk during the entire year. Some biologgers provided locations 24 h a day in some periods, but night locations were excluded. Locations were filtered at a homogeneous frequency to avoid bias in subsequent calculations: 2 h frequency to estimate home range sizes (all individuals, n = 41) and 30 min frequency to estimate travelled distances (n = 24). Locations were transformed to UTM coordinates (WGS 84, EPSG: 32630).
Two migratory juveniles were excluded from this study. These individuals did not perform wandering movements like the rest of the population; they performed trans-Saharan migrations, overwintering in western Africa (García-Macía et al. 2023). The spatial strategies of these individuals influenced by different factors and migration may have been triggered under specific conditions. Therefore, only individuals which performed wandering movements within Europe (Newton 2010) were included in this study.
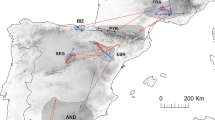
Individuals were classified into geographical regions and age classes to analyse and visualize data. We established five geographical regions following the distribution of the colonies in Spain (Del Moral 2017), also according to previous studies on the species (Hernández and Margalida 2008). Furthermore, individual data were classified as juveniles (<1 year after fledging), immatures (1–3 years old), and subadults (4 years old; Hernández and Margalida 2008). Cinereous vultures often breed for the first time at the age of 5 years (Cramp and Simmons 1980), but occasional examples of reproduction during the fourth or even third year were confirmed (Tewes 1996). We did not have field confirmation of breeding events, so we considered that individuals reached adulthood at the age of 5 years.
Calculation of movement metrics
Some movement metrics were calculated to study the wandering movements during the juvenile dispersal period of cinereous vultures.
First, distance travelled was calculated (kilometrekm), that is, the Euclidian distance between locations, which provides information on the degree of mobility and flight energetic effort (Morant et al. 2023). We calculated monthly accumulated distance as the sum of all distances travelled during each calendar month. This variable was only calculated using individuals tagged with high-frequency GPS biologgers (2 locations per hour; n = 23), excluding individuals with low-frequency emitters, which could greatly distort the calculation of this metric. Second, we estimated the mean distance to nest during each month (n = 40). We selected the farthest location available during each day and calculated the Euclidian distance to the natal nest. Finally, we calculated the monthly average. This variable provides valuable information on the distance of the areas where movements are performed and the degree of relationship with the colony of origin during different stages of the lifecycle (García-Macía et al. 2022a). Both accumulated distances and distances to nest were estimated with the function ‘step_lengths’ from ‘amt’ R library (Signer et al. 2019). Third, monthly home range sizes (95% KDE) and core areas (50% KDE) were estimated to determine the area occupied by the individual during each month (n = 40). Both 95% and 50% kernel density estimators (KDE) were calculated with the function ‘kernelUD’ from ‘adeHabitat’ R library (Calenge 2006). We used the ad hoc method to estimate the smoothing parameter (Schuler et al. 2014).
Statistical analyses
A series of generalized linear mixed models (GLMMs) were performed using the function ‘glmer’ from lme4 package in R Software v. 4.0.5. Accumulated distances, distances to nest and home ranges (95% KDE) were fitted as response variables (50% KDE was excluded after multicollinearity test with the functions ‘varclus’ and ‘redun’ from Jmisc R package; Harrell 2020), while the three following measures were fitted as explanatory variables: 1) sex (male, female); 2) age class (juvenile, immature, sub-adult); and 3) season (spring, summer, autumn and winter). All models were run including individual identification as a random effect. We run models with all pairwise interactions among variables (sex*age, sex*season and age*season).
Models were compared using the Akaike Information Criterion (AIC; Burnham and Anderson 2002) using the functions ‘model.sel’ from MuMIn R library (Bartón 2009). The selected model was the one with the lowest AIC value (ESM Table S2). When there was no single best model (delta values < 2; Burnham and Anderson 2002), a multi-model averaging over a set of best ranked models was carried out to calculate the explanatory variables’ effects, using the function ‘model.avg’ from MuMIn R library.
AIC model selection was also performed to determine the distribution of the response variables (Gamma, Poisson, or Gaussian). Gamma distributions with ‘log’ link functions were selected for all response variables (residuals plots appear in Fig. S1).
Maps were drawn with QGIS 3.16.6. A significance level for the explanatory variables’ effects was established at < 0.05.
Results
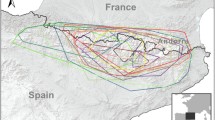
The 40 young cinereous vultures tracked in this study moved throughout the Iberian Peninsula and southern Europe during the juvenile dispersal period (until the 5 year; Fig. 1). Individuals from the Center-South and West (relating to the Iberian Peninsula) mainly explored the southeastern sector of the Iberian plateau; those from the Center covered the core of the peninsula, with frequent incursions to the north, south and east; northern individuals often performed movements into the northern and southern mountains; and individuals from the northeast region not only mainly explored that region but also southern Europe (mainly France, Italy and Germany).
The best models predicting home range sizes (95% kernel density estimators; KDE), accumulated distances and distances to nest were those containing all factors (sex, season and age) and the interaction between age and season (the weights of these models were close to 1; Table S1): y = sex + age + season + age*season.
Females occupied larger home ranges (95% KDE), averaging 17,190 ± 54,903 km2, than males (x̄ = 6,456 ± 17,714 km2), a pattern also reflected in core areas (Table 1 and S1, Fig. 2). The median distance to the nest of females was approximately 3.7 times higher than that of males (52 vs. 14 km). The model with monthly accumulated distances did not report differences between sexes, but this variable also tended to be higher in females (x̄ = 1757 ± 1131 km) than in males (x̄ = 1437 ± 842 km).
Age and season, and especially the interaction between these two factors, also influenced the movement metrics of young cinereous vultures. Most of the differences were between juveniles and the other two age classes during the spring and summer months (Table 2); although, in these seasons all age classes tended to travel more distance (Fig. 4). Juveniles occupied larger areas, moved farther from the natal nest, and travelled more distance than immatures and subadults during spring and summer, but not during autumn and winter, when the values of all variables were low and similar between age classes (Figs. 3 and 4). Immatures also travelled more distance per month than subadults during spring and summer, but no differences were found for home range sizes and distances to the nest (Tables 1 and 2, Fig. 4). Specifically, the median value of the home range size of juveniles in spring was 7.6 times higher than that of immatures, and 18.2 times than that of subadults. The median value of the accumulated distances of juveniles during spring was 1.7 times higher than that of immatures, and 2.8 times than that of subadults. The median value of the distances to the nest for juveniles during spring was 1.8 times higher than that of immatures, and 2.4 times than that of subadults. These differences, although slightly smaller, were also found during summer (see Fig. 4).
Discussion
This study is, to the best of our knowledge, the first to investigate the wandering movements of Iberian cinereous vultures during the juvenile dispersal period. Some movement metrics (accumulated distance, distance to nest and KDEs) were used to analyse the influence of sex, age and season in the wandering movements of the species during this period. Results presented in this work may be considered in future management plans and studies on this species and other soaring scavengers.
Female cinereous vultures occupied larger home ranges and flew farther from their natal nest than males, consistently within age classes. Furthermore, females also tended to travel more than males, which implies greater flight effort. The cinereous vulture is considered a monomorphic species, but females are slightly bigger than males (Cramps and Simmons 1980). The hierarchical exploitation of resources in European vultures is influenced by both age and body size (Moreno-Opo et al. 2020). Therefore, it was expected that female cinereous vultures were dominant over males (Van Overveld et al. 2018) with the latter being forced to explore further locations. However, our models did not support this hypothesis, in accordance with other studies of the movement ecology of large scavengers in Iberia (Morant et al. 2023). The higher feeding requirements of females may be one of the causes of their greater wandering movements. Spatial segregation between sexes might also be one of the mechanisms used by the species for an optimal exploitation of carrion, an unpredictable and highly demanded resource. Another plausible hypothesis is that females travel more distances to search for a future breeding site (Literák et al. 2022). More specific studies on the dominance hierarchy between sexes in the cinereous vultures are necessary to test these hypothesis.
Juveniles performed farther wandering movements and occupied larger areas than older vultures. As individuals reach adulthood, they performed more predictable and shorter movements within a stable territory. This trend is very common among raptor species, which usually perform more efficient and regular movements as they reach adulthood (Newton 1982; Ferrer 1993; Balbotín and Ferrer 2009; García-Macía et al. 2022a), which largely mitigate energetic costs during juvenile dispersal, a very high-demanding period (Klarevas-Irby et al. 2021). For the specific case of a long-lived scavenging raptor such as the cinereous vulture, age spatial segregation may be driven by the dominance hierarchy during carrion exploitation. There is a dominance gradient from the adults to juveniles in European vultures, following an age and body size-based linear pattern (Svanbäck and Bolnick 2005; Moreno-Opo et al. 2020). Therefore, juvenile vultures, unable to access carrion given the higher hierarchy of the elders, are forced to forage throughout larger and more distant areas in order to avoid competition. Furthermore, these dynamics may be also related to progressive settlement in a breeding site: older birds (subadults) may perform shorter movements close to birthplaces as they are trying to reproduce. These juvenile dispersal dynamics and hierarchical organization, which combine philopatry, large wandering movements in the younger birds, and colonial adhesion in the elders may entail some population advantages. First, the spatial segregation between age classes in the foraging activity may reduce intra-specific competition for carrion, a limited resource that is clumped, unpredictable, and first accessed by adults (Bosè et al. 2012). Second, these spatial ecological patterns allow the adhesion of the colony and the maintenance of colonial territories beyond generations, but young birds could also be pioneers (Lees and Gilroy 2021) in exploring new areas that ultimately expand or modify the territory of the colony.
Season also influenced the wandering movements of vultures. The individuals travelled more distance during spring and summer, which may be explained by the environmental conditions. The thermal currents and wind conditions affect large-scale patterns of space use, especially in large soaring birds (Ruxton and Houston 2004; Williams et al. 2020). Therefore, vultures are able to fly more distance without increasing energy costs during warm months. On the other hand, juveniles (< 1 year old) only occupied large areas and fly farther during spring and summer, while these variables were lower during autumn and winter, and similar for all age classes. Therefore, juveniles are expected to stay near the natal nest during their first months of life, and to perform high-demanding large movements far from the natal nest and occupy larger areas during their first spring and summer. From the second year of life onwards, seasonal differences are diluted as the movements of individuals are confined to known areas and their behaviour is, therefore, more predictable and repetitive. From a conservation perspective, the months of increased wandering movements are the most dangerous for non-experienced birds (Oppel et al. 2015), so this period is critical for populations’ conservation.
The great inter-individual variability observed within our sample needs to be highlighted: the models and variables used in this study (sex, age and season) did not explain by themselves all the variability of the wandering movements of the cinereous vulture during the juvenile dispersal period. Future studies should investigate the influence of other no tested factors, such as landscape, presence and abundance of supplementary feeding sites or dumps, colony size, interspecific interactions, stochastic events, etc. However, this work provides a basic approach to the juvenile dispersal of the cinereous vultures in Iberia and demonstrates the influence of both intrinsic (sex and age) and extrinsic factors (season) in the wandering movements of large scavengers during the juvenile dispersal period.
Data availability
GPS fixes are available in https://movebank.org/ under author’s permissions.
References
Balbontín J, Ferrer M (2009) Movements of juvenile Bonelli’s eagles Aquila fasciata during dispersal. Bird Study 56:86–95
Bartoń K (2009) MuMIn: Multi-model inference (R package version 0.12.2).
BirdLife International (2021) Aegypius monachus. The IUCN Red List of Threatened Species 2021: e.T22695231A154915043. https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T22695231A154915043.en
Bosè M, Duriez O, Sarrazin F (2012) Intra-specific competition in foraging Griffon Vultures Gyps fulvus: 1 Dynamics of group feeding. Bird Study 59:182–192. https://doi.org/10.1080/00063657.2012.658639
Burnham KP, Anderson DR (2002) Burnham KP, Anderson DR (2002) Model selection and multimodel inference: A practical information-theoretic approach (2ª) Ecological Modelling. Springer Science & Business Media
Calenge C (2006) The package adehabitat for the R software: tool for the analysis of space and habitat use by animals. Ecol Modell 197:1035
Carrete M, Donázar JA (2005) Application of central-place foraging theory shows the importance of Mediterranean dehesas for the conservation of the Cinereous Vulture, Aegypius monachus. Biol Conserv 126:582–590. https://doi.org/10.1016/j.biocon.2005.06.031
Costillo E, Corbacho C, Marón R, Villegas A (2007) Diet plasticity of Cinereous Vulture Aegypius monachus in different colonies in the Extremadura (SW Spain). Ardea 95(2):201–211
Cramp S, Simmons KEL (1980) Handbook of the Birds of Europe the Middle East and North Africa The Birds of the Western Palearctic Hawks to Bustards, vol II. Oxford University Press, Oxford
Del Moral JC (2017a) El buitre negro en España, población reproductora en 2017 y método de censo. SEO/BirdLife, Madrid
Del Moral JC, De la Puente J (2017b) Buitre negro – Aegypius monachus. In: Salvador A, Morales MB (eds) Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales, Madrid
Ellegren H (1996) First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc Royal Soc B 263:1635–1641
Ferrer M (1993) Juvenile dispersal behaviour and natal philopatry of a long-lived raptor, the Spanish imperial eagle Aquila adalberti. Ibis 135:132–138
Forsman ED, Anthony RG, Reid JA, Loschl PJ, Sovern SG, Taylor M, Biswell BL, Ellingson A, Meslow EC, Miller GS, Swindle KA, Thrailkill JA, Wagner FF, Seaman DE (2002) Natal and breeding dispersal of northern spotted owls. J Wildl Manag 149:1–35
García-Macía J, López-Poveda G, De La Puente J, Bermejo-Bermejo A, Galán M, Álvarez E, Morollón S, Urios V (2022a) The variability of juvenile dispersal in an opportunistic raptor. Curr Zool zoac. https://doi.org/10.1093/cz/zoac039
García-Macía J, Vidal-Mateo J, De La Puente J, Bermejo A, Urios V (2022b) Spatial ecology of the Red Kite (Milvus milvus) during the breeding period in Spain. Ornis Fenn 99:150–162
García-Macía J, Gálvez M, Plana G, Vallverdú N, Álvarez E, Galán M, Iglesias-Lebrija JJ, Urios V (2023) Analysis of the trans-Saharan migration and wintering areas of GPS-tagged Cinereous Vultures Aegypius monachus. Bird Study. https://doi.org/10.1080/00063657.2023.2274312
Gavashelishvili A, McGrady M, Ghasabian M, Bildstein KL (2012) Movements and habitat use by immature Cinereous Vultures (Aegypius monachus) from the Caucasus. Bird Study 59:449–462. https://doi.org/10.1080/00063657.2012.728194
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Ann Rev Ecol, Evol Syst 13:1–21
Harrell FE (2020) Hmisc: Harrell miscellanous. R package version 4.4-0. https://cran.r-project.org/web/packages/Hmisc/index.html
Hernández M, Margalida A (2008) Pesticide abuse in Europe: Effects on the Cinereous vulture (Aegypius monachus) population in Spain. Ecotoxicology 17:264–272. https://doi.org/10.1007/s10646-008-0193-1
Hiraldo F (1976) Diet of the Black Vulture (Aegypius monachus) in the Iberian peninsula. Acta Vertebrata 3:19–31
Hiraldo F (1983) Breeding biology of the Cinereous Vulture. In: Wilbur SR, Jackson JA (eds) Vulture Biology and Management. University of California Press, Berkeley, p 1
Iglesias-Merchán C, Diaz-Balteiro L, De la Puente J (2016) Road traffic noise impact assessment in a breeding colony of Cinereous Vultures (Aegypius monachus) in Spain. J Acoust Soc Am 139:1124–1131. https://doi.org/10.1121/1.4943553
Kang JH, Hyun BR, Kim IK, Lee H, Lee JK, Hwang HS, Eom TK, Rhim SJ (2019) Movement and home range of Cinereous Vulture Aegypius monachus during the wintering and summering periods in East Asia. Turk Zool Derg 43:305–313. https://doi.org/10.3906/zoo-1807-3
Kenward RE (2001) A manual for wildlife radio tagging. Academic Press, London
Kim JH, Chung OS, Lee WS, Kanai Y (2007) Migration routes of Cinereous Vultures Aegypius monachus in northeast Asia. J Raptor Res 41:161–165. https://doi.org/10.3356/0892-1016(2007)41[161:MROCVA]2.0.CO;2
Klarevas-Irby JA, Wikelski M, Farine DR (2021) Efficient movement strategies mitigate the energetic cost of dispersal. Ecol Lett 24:1432–1442. https://doi.org/10.1111/ele.13763
Krüger O (2005) The evolution of reversed sexual size dimorphism in hawks, falcons and owls: a comparative study. Evol Ecol 19:467–486. https://doi.org/10.1007/s10682-005-0293-9
Lees AC, Girloy JJ (2021) Bird migration: When vagrants become pioneers. Curr Biol 31:R1568–R1570. https://doi.org/10.1016/j.cub.2021.10.058
Literák I, Raab R, Škrábal J, Vyhnal S, Dostál M, Matušík H, Makoň K, Maderič B, Spakovszky P (2022) Dispersal and philopatry in Central European Red Kites Milvus milvus. J Ornithol 163:469–479. https://doi.org/10.1007/s10336-021-01950-5
López-López P, Perona A, Egea-Casas O, Etxebarria Morant J, Urios V (2021) Tri-axial accelerometry shows differences in energy expenditure and parental effort throughout the breeding season in long-lived raptors. Curr Zool. https://doi.org/10.1093/cz/zoab010
Maness TJ, Anderson DJ (2013) Predictors of Juvenile Survival in Birds. Ornithol Monogr 78:1–55. https://doi.org/10.1525/om.2013.78.1.1
Martínez JE, Zuberogoitia I, Escarabajal JM, Cerezo E, Calvo JF, Margalida A (2020) Breeding behaviour and time-activity budgets of Bonelli’s eagles Aquila fasciata: marked sexual differences in parental activities. Bird Study 67:35–44. https://doi.org/10.1080/00063657.2020.1733487
Morán-López R, Sánchez JM, Costillo E, Corbacho C, Villegas A (2006) Spatial variation in anthropic and natural factors regulating the breeding success of the Cinereous Vulture (Aegypius monachus) in the SW Iberian Peninsula. Biol Conserv 130:169–182. https://doi.org/10.1016/j.biocon.2005.12.011
Morant J, Arrondo E, Sanchez-Zapata J, Donázar JA, Cortés-Avizanda A, De la Riva M, Blanco G, Martínez F, Oltra J, Carrete M, Margalida A, Oliva-Vidal P, Martínez JM, Serrano D, Pérez-García JM (2023) Large-scale movement patterns in a social vulture are influenced by seasonality, sex, and breeding region. Ecol Evol 13:e9817. https://doi.org/10.1002/ece3.9817
Moreno-Opo R, Arrerondo Á, Guil F (2010) Foraging range and diet of Cinereous Vulture Aegypius monachus using livestock resources in central Spain. Ardeola 57:111–119
Moreno-Opo R, Trujillano A, Margalida A (2020) Larger size and older age confer competitive advantage: dominance hierarchy within European vulture guild. Sci Rep. https://doi.org/10.1038/s41598-020-59387-4
Muñoz-Adalia EJ, Hernández A (2013) El buitre negro en España: Una revisión de su biología y estado de conservación. Chronica Natura 3:66–75
Newton I (2010) Bird Migration. William Collins, Glasgow
Newton I, Marquiss M (1982) Fidelity to Breeding Area and Mate in Sparrowhawks Accipiter nisus. J Anim Ecol 51:327. https://doi.org/10.2307/4327
Newton I, Davis PE, Davis JE (1989) Age of first breeding, dispersal and survival of red kites Milvus milvus in Wales. Ibis 131:16–21
Oppel S, Dobrev V, Arkumarev V, Saravia V, Bounas A, Kret E, Velecski SS, Nikolov SC (2015) High juvenile mortality during migration in a declining population of a long-distance migratory raptor. Ibis 157:545–557. https://doi.org/10.1111/ibi.12258
Panter CT, Amar A (2022) Using web-sourced photographs to examine temporal patterns in sex-specific diet of a highly sexually dimorphic raptor. R Soc Open Sci. https://doi.org/10.1098/rsos.220779
Ruxton GD, Houston DC (2004) Obligate vertebrate scavengers must be large soaring fliers. J Theor Biol 228:431–436. https://doi.org/10.1016/j.jtbi.2004.02.005
Schuler KL, Schroeder GM, Jenks JA, Kie JG (2014) Ad hoc smoothing parameter performance in kernel estimates of GPS-derived home ranges. Wildlife Biol 20:259–266. https://doi.org/10.2981/wlb.12117
Signer J, Fieberg J, Avgar T (2019) Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol Evol 9:880–890. https://doi.org/10.1002/ece3.4823
Svanbäck R, Bolnick DI (2005) Intraspecific competition affects the strength of individual specialization: an optimal diet theory method. Evol Ecol Res 7:993–1012
Tewes E (1996) The European Black Vulture (Aegypius monachus L.), management techniques and habitat requirements. PhD Dissertation, University of Vienna, Austria
Van Overveld T, García-Alfonso M, Dingemanse NJ, Bouten W, Gangoso L, de la Riva M, Serrano D, Donázar JA (2018) Food predictability and social status drive individual resource specializations in a territorial vulture. Sci Rep. https://doi.org/10.1038/s41598-018-33564-y
Walls S, Kenward R (1995) Movements of radio-tagged common buzzards Buteo buteo in their first year. Ibis 137:177–182
Whitfield DP, Douse A, Evans RJ, Grant J, Love J, McLeod DRA, Reid R, Wilson JD (2009) Natal and breeding dispersal in a reintroduced population of white-tailed eagles Haliaeetus albicilla. Bird Study 56:177–186. https://doi.org/10.1080/00063650902792023
Williams HJ, Shepard ELC, Holton MD, Alarcón PAE, Wilson RP, Lambertucci SA (2020) Physical limits of flight performance in the heaviest soaring bird. PNAS. https://doi.org/10.1073/pnas.1907360117/-/DCSupplemental
Yamaç E, Bilgin CC (2012) Post-fledging movements Cinereous Vultures Aegypius monachus in Turkey revealed by GPS telemetry. Ardea 100:149–156. https://doi.org/10.5253/078.100.0206
Acknowledgements
We would like to thank the Government of Aragon (SARGA), the Cos d’Agents Rurals de Catalunya (CAR), and the Grup de Suport de Muntanya for their collaboration in the marking of the Caça de Boumort National Reserve. We are also grateful for the effort made by the Natural Environment Agents from Extremadura in the monitoring of the cinereous vulture population and their collaboration in the captures and taggings, especially to their Vertical Work Team; and for the work done by the Center for Wildlife Recovery and Environmental Education "Los Hornos" and the Wildlife Hospital of AMUS.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. We would like to thank the following entities for providing the data and funding the projects: Iberdrola España Foundation (MIGRA program of SEO/BirdLife), Asociación Trenca, Generalitat de Catalunya, Junta de Extremadura, GREFA (supported by REDEIA, MITERD, Junta de Castilla y León, Junta de Comunidades de Castilla la Mancha and Comunidad de Madrid), Cabañeros National Park and Sierra de Guadarrama National Park.
Author information
Authors and Affiliations
Contributions
JGM compiled the data, designed the study, performed the analyses and wrote the original draft. EA, MG, JJI-L, MG, GP, and NV were involved in tagging processes, field work, data compilation and project management. VU reviewed the original draft and supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This paper is part of Jorge García-Macía’s PhD thesis.
Ethical approval
The experiments comply with the current laws of Spain and taggings were carried out with the permission of the administration.
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Macía, J., Álvarez, E., Galán, M. et al. Age, season and sex influence juvenile dispersal in the Iberian cinereous vultures (Aegypius monachus). J Ornithol 165, 325–335 (2024). https://doi.org/10.1007/s10336-023-02126-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02126-z