Abstract
Most farmland birds experience strong declines across Europe. These declines are typically associated with agricultural intensification but research on alternative local causes remains scarce. We investigated variation in reproductive success as a potential driver for the observed population declines in a fragmented population of the Meadow Pipit Anthus pratensis, a representative inhabitant of extensively managed mountain grasslands across Europe. Intense nest surveys in the entire Meadow Pipit metapopulation of the Northern Black Forest (SW Germany) between 2020 and 2022 provided information on reproductive success for 53 females distributed across nine habitat patches along an 18 km ridge of the Northern Black Forest. Hatching dates delayed by approx. 5.0 days per 100 m altitude and were almost 10 days later in a year with cold and rainy spring weather. Mean reproductive success per female and year (3.45 fledglings) was low compared to literature values (approx. 4.5) and may thus drive ongoing population declines. Mayfield nest survival estimates (approx. 51% across the nesting period) were comparably high, with most nest failures linked with predation or adverse weather. Low reproductive success further associated with comparably small clutch sizes and low fractions of second broods in habitat patches characterized by homogeneously dense swards. We suggest that restoration through extensive permanent cattle grazing coupled with succession control may be a key factor to increase population productivity.
Zusammenfassung
Variation im Fortpflanzungserfolg einer fragmentierten Wiesenpieper-Population: Spielen Unterschiede in der Vegetationsstruktur eine Rolle?
Die meisten Offenlandvögel in Europa zeigen in jüngster Zeit starke Bestandsrückgänge, die insbesondere auf die Intensivierung der Landwirtschaft zurückgeführt werden. Untersuchungen zu alternativen Rückgangsursachen sind allerdings selten. Wir untersuchten Unterschiede im Fortpflanzungserfolg als mögliche Ursache für den beobachteten Rückgang einer fragmentierten Population des Wiesenpiepers Anthus pratensis, einer typischen Vogelart extensiv genutzter Grünlandflächen in Europa. Eine intensive Nestersuche zwischen 2020 und 2022 lieferte Informationen über den Bruterfolg von 53 Weibchen in neun inselartigen Offenland-Habitaten entlang eines 18 km langen Höhenrückens im Nordschwarzwald. Der Schlupfzeitpunkt verzögerte sich um etwa 5 Tage pro 100 Höhenmeter und lag in einem Jahr mit kalter und regnerischer Witterung etwa 10 Tage später. Der Fortpflanzungserfolg pro Weibchen und Jahr (3.45 flügge Jungvögel) war im Vergleich zu Literaturwerten (ca. 4.5) relativ gering und könnte daher eine der Ursachen für den anhaltenden Rückgang der Population sein. Die nach der Mayfield-Methode ermittelte Überlebensrate der Nester (ca. 51%) war vergleichsweise hoch, wobei die meisten Nestverluste durch Prädation oder ungünstige Witterungsbedingungen verursacht wurden. Der geringe Fortpflanzungserfolg war zudem durch relativ kleine Gelegegrößen und geringe Anteile an Zweitbruten in Teilflächen mit dichter und homogener Krautschicht gekennzeichnet. Um den Fortpflanzungserfolg der Population zu erhöhen, halten wir auf Basis der Ergebnisse eine extensive Beweidung mit Rindern sowie ein Zurückdrängen der Gehölze für besonders zielführend.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population declines are documented for many European bird species (Keller et al. 2020; Burns et al. 2021) but are particularly pronounced among the inhabitants of open agricultural landscapes (Bauer et al. 2019; Kamp et al. 2020; PECBMS 2023). Farmland bird declines are typically attributed to the pervasive agricultural intensification during recent decades (Donald et al. 2006; Newton 2004). Yet, similar declines also occur in mires, heathlands, coastal areas, and other habitats that suffer far less, or more indirectly, from land use intensification (Menke 2015; Förschler et al. 2016a). Research on alternative local causes of population declines, however, remains scarce.
In our study, we assessed associations between local land use variables other than agricultural intensification and the reproductive success of a representative inhabitant of extensively managed moist grasslands, the ground nesting insectivorous Meadow Pipit Anthus pratensis (Glutz von Blotzheim and Bauer 1985). Its European population declined by approx. 63% between 1980 and 2021 (European Bird Census Council 2022), the German population by approx. 60% in just half that time interval between 1990 and 2009 (Gedeon et al. 2014) with signs for stabilisation on a low level since 2010 (Kamp et al. 2020). In the south German federal state of Baden-Württemberg, the Meadow Pipit rates as “critically endangered” (Kramer et al. 2022) given a population decline from about 600 territories around 1995 (Hölzinger and Ebenhöh 1999) to 120–160 territories in 2012–2016 (Kramer et al. 2022). The highly fragmented population today concentrates in just three strongholds in the Southern Black Forest, the Northern Black Forest, and at Lake Federsee (Gedeon et al. 2014), each isolated from their nearest neighbouring population by at least 70 km. Such small and fragmented populations are meanwhile typical for several farmland bird species within the intensively used agricultural landscape of SW Germany (e.g., Anthes et al. 2017; Seidt et al. 2017; Einstein et al. 2021).
In the Northern Black Forest, Meadow Pipits inhabit raised bogs and extensively used grassland (heathland) that is restricted by traditional land use to mountain tops at 900 to 1200 m a.s.l. (Förschler et al. 2016b). The local population size declined from 85 territories in 1995–1997 to 28 territories in 2015 for still unknown causes (Förschler et al. 2016a). Agricultural intensification, as discussed as the primary factor for Meadow Pipit declines in general (e.g., Gedeon et al. 2014; Keller et al. 2020; BirdLife International 2021), does not qualify as a local factor in the absence of agricultural intensification during the last decades. Yet, no earlier study has investigated local breeding biology in detail to better understand potential alternative drivers of population declines.
We, therefore, focussed on reproductive success (= total female productivity) as one key candidate cause for local population declines, as suggested previously for other farmland bird species (Donald et al. 2002; Boatman et al. 2004; Plard et al. 2020). From nest monitoring data of almost the entire Meadow Pipit population between 2020 and 2022, we first analysed variation in breeding phenology between years and along the investigated altitudinal gradient as shown for Meadow Pipits in Great Britain (Coulson 1956). Second, we investigated the degree to which clutch size and reproductive success varied between brood types, grassland patches and study years. Finally, we document reasons for nest failure and nestling mortality, quantify nest survival rates, and derive suggestions for targeted conservation measures.
Methods
Study area
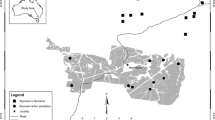
Our study took place in the “Grindenschwarzwald” in the Northern Black Forest range (Fig. 1, 48°32 N, 8°13 E, Germany). “Grinden” are a regional type of semi-open grassland (mountain heathland) that is embedded in extensive coniferous forests. The “Grinden” heathlands originated from deforestation or partial land clearance by burning, followed by livestock grazing and litter use between the sixteenth and nineteenth century (Förschler et al. 2016b). They are part of the annex I habitat type 4030—European dry heaths and thus protected under the NATURA 2000 Habitats Directive (Olmeda et al. 2020). To preserve this habitat type with its highly diverse and threatened flora and fauna, low-intensity grazing was reintroduced on small grassland patches in 1995, but the number of herds and area coverage continuously expanded thereafter. Beyond the “Grinden”, grassland patches in the study area are restricted to mowed or mulched ski slopes. Surveys in the current study focused on all (seven) grassland patches for which Meadow Pipit breeding was confirmed or suggested during a recent large-scale survey (Förschler et al. 2016a) but also included another ten grassland patches with historic breeding that had recently been rated unoccupied (Fig. 1). Grassland patches in the nearby valleys were abandoned by Meadow Pipits several decades ago (Förschler et al. 2016a) and thus not investigated.
For statistical comparisons, we combined the individual grassland patches into three grassland patch groups: Hornisgrinde North plus Hornisgrinde South into Hornisgrinde, Schliffkopf plus Großer Geißkopf into Schliffkopf, and all other grassland patches into “Others” given otherwise small sample size (Fig. 1).
Nesting survey
We visited each grassland patch about once per week during the pre-breeding season, starting with the arrival of Meadow Pipits in March, and the entire breeding season until early August in three successive years 2020–2022. Pre-breeding visits were necessary since Meadow Pipits exhibit intense song displays during the first days after arrival but become rather cryptic once pair bonds have been established (Südbeck et al. 2005) so that territories can easily be overlooked. In the core breeding season between early May and August, surveys focussed on nest building, incubating, and food provisioning adult birds, spending at least half an hour per territory in that Meadow Pipit presence was confirmed during the pre-breeding visits. From these weekly visits, we extracted the number of breeding pairs (pairs with confirmed nesting) and the number of solitary males (males with intense and continuous singing activity without confirmed pairing or nesting).
To localize nests, we pursued adult birds with nesting material, when returning to nests during incubation, or with nestling food items from typically > 60 m distance to minimize disturbance. Nest sites localized within approximately ± 4 m were carefully approached and revisited about every third day to document nest position, nest success and the number of fledglings. After fledging or nest failure, we continued surveys to detect replacement or second broods. Causes for nest failure were inferred from traces in and around the nest, the presence and behaviour of the adults, and weather conditions on the days preceding nest failure. For each nest, we documented the presumed initial clutch size (i.e., the largest number of eggs documented, or the number of nestlings plus unhatched eggs), the number of fledglings (i.e., the number of nestlings during the last visit before fledglings could be confirmed in close nest vicinity), and assigned it—to our best knowledge—to first brood, second brood or replacement brood based on date and observation circumstances (first brood: nest of a pair for that no earlier signs of breeding attempts were available; second brood: nest of a pair for that a successful first brood was confirmed; replacement brood: nest of a pair for that a previous nest loss was documented or inferred from abrupt termination of feeding or incubation activity).
Reproductive success
Our detailed surveys allowed us to closely approximate full reproductive success as the total number of fledglings over successive broods for each female and year. This was possible because most individuals of the breeding population were individually colour-ringed with combinations of 3 colour rings (seven colours) and one metal ring. Our ringing total of 32 adult birds caught from mist nets and 157 nestlings resulted in colour-ringed adult fractions of approx. 51% in 2020, 64% in 2021, and 62% in 2022. Since colour-ringed females showed strong territory-fidelity with a given male per season, un-ringed females were also assumed identical individuals for replacement or second broods in a given male’s territory. Only in one case, a colour-ringed female changed territory and thus its partner after losing its first brood.
Unknown hatching dates were estimated based on nestling size, nestling behaviour, and feather development by comparison with local nestlings of known age and literature reports assuming a total nestling period of 13 days (Glutz von Blotzheim and Bauer 1985; Hölzinger and Ebenhöh 1999).
We obtained reproductive success values (= fledgling counts) per year for all 53 females that stayed in the breeding area well into the breeding period. For 10 of these, values represent lower bounds to true fledgling count, either because they rest on observations from a distance for one brood (then as a minimum estimate of fledged young) or because there is small chance, we missed a second or replacement brood when a female could not be followed into the late breeding period. We report findings based on the full sample below, but provide the analysis reduced to 43 females with complete information in Online Resource C.2, with near-identical qualitative (and even quantitative) findings.
Statistical analyses
All statistical analyses were implemented in R version 4.2.2 (R Core Team 2022). For linear models, we used the glmmTMB package (Brooks et al. 2017). The first model describes variation in hatching dates of first broods (n = 40 nests, Gaussian error family) along the altitudinal gradient and between study years, including their interaction. Further models quantify variation in clutch size (n = 62 clutches) and in reproductive success (i.e., fledgling counts per female, n = 53 females) between patch groups, study years and brood types (i.e., first broods versus replacement or second broods), including the PatchGroup: BroodType and Year: BroodType interactions. Both models used the generalized poisson model family (‘genpois’ with a log-link) to reflect underdispersion in their count responses. For model assessment, we inspected residuals standardized for their distribution family (independence of fitted values, homogeneity across predictor variables) and conducted posterior predictive checks on model-simulated data (dispersion, zero inflation, and distribution relative to observed data) using the routines provided by Santon et al. (2023). Mild zero inflation in the fledgling model was captured by adding a zi-formula that modelled extra zeroes by grassland patch groups.
We complement our descriptions of apparent nest success and causes of nest failure with a formal analysis of daily nest survival rates (Mayfield DSR) from a binary logistic regression on nest outcome (0 = success, 1 = failure) as implemented in MARK (White and Burnham 1999) and accessed through the R package RMark (Laake and Rexstad 2008). As above, we described DSR as a function of grassland patch group, study year and brood type, and included season and nest age as covariates because both often affect DSR (Rotella et al. 2004). From overall mean DSR, we estimated nest survival probability as DSR raised to the power of the duration of incubation and nestling stages (26 days) (Johnson 1979).
We refrain from presenting P values and their associated evaluation of binary null hypotheses in accordance with current recommendations for objective statistical reporting (Halsey et al. 2015; Berner and Amrhein 2022). Instead, we report effect size estimates with their compatibility intervals, which are identical to classic confidence intervals, but terminology shifts emphasis from trust in hypothesis testing to a description of the central 95% density interval of effect values that are most compatible with the observed data given the statistical model (Berner and Amrhein 2022).
Results
Population size
Meadow Pipits were confirmed breeding in nine grassland patches along an 18 km section of the main ridge of the Northern Black Forest (Fig. 1). Seven of these grassland patches are extensively grazed mountain heathlands, two are mulched and mowed ski slopes (Table 1). Territory numbers as well as the numbers of breeding females and solitary males varied strikingly between study years despite comparably intense survey effort. Grassland patch occupancy dynamics included three recolonisation events and one extinction event in marginal subpopulations (Table 1).
Breeding phenology
Out of 62 documented nests (Table 2), one was found during nest building, 16 during incubation, and 45 during the nestling stage. 44 nests were classified as first broods, 12 as second broods and 6 as replacement broods.
Nestlings hatched between mid-May and end of July. Lumped across study years, average hatching dates of first broods increased with nest altitude by 4.98 (95% compatibility interval, CI 2.33–7.63, Online Resource A.1) days on average per 100 m altitude, which implies hatching delays compared to the Zollstock-Heide site (950 m a.s.l.) by 3.5 days at Schliffkopf (1020 m a.s.l.) and 10.5 days at Hornisgrinde (1160 m a.s.l.). Regression slopes varied slightly between years but were consistently positive (Fig. 2, Online Resource A.2). 2021 stands out with particularly late first broods, with hatching dates approx. 10 days later than in 2020 and 2022 at medium altitude (Fig. 2, Online Resource A.2).
Clutch size and hatching success
Most clutches contained four eggs, with an overall average clutch size of 3.79 (CI 3.60–3.98). We could not detect any relevant variation in mean clutch sizes between sites, years, or brood types (Fig. 3, Online Resource B). Overall hatching rate was high, with 193 out of 222 non-predated eggs (87%) hatching. 11 of these non-hatching eggs were contributed by a single female from which all eggs of three successive broods with the same male did not develop.
Reproductive success
Fledgling counts varied between zero and nine (Fig. 4a, b). Descriptive analysis revealed an overall average of 3.45 ± 2.36 (mean ± SD) fledglings per female and year, with a particularly high value at Hornisgrinde (4.25 ± 2.47) compared to Schliffkopf (2.64 ± 1.95) and Others (2.93 ± 2.25), and a particularly low value in 2021 (2.71 ± 2.08) compared to 2020 (4.10 ± 2.57) and 2022 (3.44 ± 2.25). We explored possible reasons for these differences through a formal analysis of the effects of year, brood type, and grassland patch groups. Average reproductive success per female was strikingly linked to brood type, where females with only a single brood had clearly lower average reproductive success than those with a replacement or second brood (Fig. 4a). Brood type effects did not vary among years (Fig. 4a, Online Resource C.1), so that the low average productivity in 2021 cannot be explained by low average nest success, but associated with a low fraction of females that initiated a second brood in that year (Fig. 4c). Average reproductive success of single-brooded females remained stable across patch groups (Fig. 4b, Online Resource C.1). In contrast, the benefit for females with 2nd breeding attempts compared to single-brooded females varied strikingly among patch groups (Fig. 4b, Online Resource C.1), with almost twice the reproductive success at Hornisgrinde but far lower productivity benefit in the other two patch groups (within-site contrasts in Online Resource C.1). This difference goes along with 2nd breeding attempts at Hornisgrinde constituting largely of true second broods (after completed first broods), while those at the other patch groups largely relating to replacement broods after first brood failure (Fig. 4d).
Variation in the number of fledglings per female between years (a) and grassland patch groups (b), and fractions of brood types for years (c) and grassland patch groups (d). Bold markers indicate predicted means and error bars their 95% compatibility intervals. For statistical details see Online Resource C
Nestling mortality and nest survival
14 nests (23% of 62 nests) failed before fledging for variable reasons between years (Table 3). Six nest losses could be associated with predation (nestlings or eggs depredated, parents alive), three with adverse weather (nestlings dead but without apparent damage in nest during cold and rainy weather period, parents alive), and two with the loss of a parent (nestlings dead in nest, only one parent present: 1 confirmed roadkill of female, 1 female disappeared for unknown reason). In one case of depredation, the predator was identified as a small carnivore (marten or weasel) from bite marks on colour rings found in the nest. Beside carnivores, potential nest predators in the study area include birds of prey, corvids, or European adders Vipera berus.
One nest out of two found on a ski slope was rescued from destruction by mowing through a targeted late mowing arrangement. Another likely loss was prevented by fencing the nest before intense sheep grazing. By contrast, none of five nests recorded in low intensity grazing cattle pastures was damaged by livestock trampling.
Overall daily nest survival was 0.9744 ± 0.0067 SE (95% CI 0.9572–0.9848), resulting in a mean probability of 0.510 (95% CI 0.321–0.672) for nests to survive the entire 26-day nesting period (13 days incubation, 13 days nestling period). Variation in daily survival rate was best explained by a grassland patch group model, with highest mean DSR at Hornisgrinde (Fig. 5a, Online Resource D). Nest age was the only other predictor that came close in predictor power to the intercept-only model (Online Resource D), possibly indicating a modest increase in DSR with nest age (Fig. 5b).
Estimated Mayfield daily survival rates for Meadow Pipit nests and their variation among grassland patch groups (left, showing the global mean DSR as dashed line) and with nest age spanning the 26-day period of incubation (13 days) and nestling feeding (13 days) (right), including 95% compatibility intervals. See Online Resource D for model comparisons
Discussion
We studied nesting ecology and reproductive success in a continuously declining, fragmented grassland population of Meadow Pipits in the Northern Black Forest in 2020–2022. Hatching dates of first broods were earlier in lower altitudes and varied between study years. Clutch sizes showed low variation and associated with neither year, patch group nor brood type. Reproductive success per female varied strikingly between grassland patch groups and was largely driven by nest survival and the fraction of females that raised second broods.
The observed increase in hatching dates with altitude matches previous findings but was more pronounced compared to Britain populations where average hatching dates increased by only 2.5 days per 100 m altitude (Coulson 1956). Local habitat conditions may have intensified the altitudinal effect in our study area: our highest altitude patch group, Hornisgrinde, is characterized by a raised bog, where cool and moist microclimate may favour particularly late hatching. Hatching date differences between years correlate well with weather conditions, where particularly late hatching in 2021 was associated with a cold spring and snowfall until May. Mean air temperatures in May were 10.9 °C and 13.3 °C in 2020 and 2022, but only 7.9 °C in 2021 at a nearby weather station at 800 m a.s.l. (Wetterdienst 2022).
The documented average clutch size of 3.79 eggs per nest was low compared to literature values that vary between 3.89 and 5.4, depending on study region (Davies 1958; Constant and Eybert 1980; Hötker and Sudfeldt 1982; Rose 1982; Hölzinger and Ebenhöh 1999; Malm et al. 2020). However, clutch size has also been described to increase with latitude (Hötker and Sudfeldt 1982), and the Northern Black Forest is close to the southern range margin of Meadow Pipit (Keller et al. 2020). Average clutch sizes in more southern populations were still slightly higher, with 4.15 in the Southern Black Forest during 1981–1996 (Hölzinger and Ebenhöh 1999) and 4.40 in the Swiss Jura during 1972–1974 (Pedroli 1978). Given a lack of previous data from the Northern Black Forest we cannot assess, however, whether current average clutch sizes are lower compared to the mid-twentieth century when local Meadow Pipit populations were considered stable.
Our reproductive success data are difficult to compare to literature values since reproductive success obviously differs between years, and demography as well as life history strategies may differ strongly between populations (Barras et al. 2021). Yet, it is striking that average reproductive success per female and year in our study area was about one fledgling below that reported from Lower Saxony (Northern Germany) with 4.45 raised juveniles per year and female over a 5-year period (Hötker and Sudfeldt 1982). The authors hint at a large fraction of second and even third broods and estimate 2.3 broods or breeding attempts per female and year (Hötker and Sudfeldt 1982), which compares to only 1.4 breeding attempts per female and year in our study. This indicates that low reproductive success in the Black Forest, which goes along a lack of (successful) second and third broods, is insufficient to maintain population stability.
Differences in reproductive success between years might be explained by the cold climatic conditions in 2021, where delayed first broods and a shortage of second broods directly contributed to the low average reproductive success compared to 2020 and 2022. Adverse weather conditions thus not only directly cause nest failures, but also indirectly reduce reproductive success through a reduction in successive brood numbers (e.g., Frey 1989; Förschler et al. 2005). Such effects may intensify given that weather (and rainfall) extremes are predicted to become more frequent (e.g., Seneviratne et al. 2012; Zeder and Fischer 2020).
Apparent nest success (77%) was higher than that reported for four different years in Scotland (range: 18–65%, Malm et al. 2020) and for a population in Poland (71%, Halupka 1998a). Also, our overall Mayfield estimate for nest survival of 50.9% (49.6% when adjusted to a 27-day period) was similar to the 48.2% (27-day period) reported from a population in Poland (Halupka 1998a). While these findings may suggest nest losses as a minor reason for low productivity in the Northern Black Forest population, we also found a striking link between nest survival and the high reproductive success per female at the Hornisgrinde patch group, which grounded in a combination of generally higher nest survival and—to some extent as a consequence of higher nest survival of first broods—a larger fraction of females that raise second broods. This finding is indeed opposite to the expectation of larger fractions of second broods at lower altitudes where an earlier onset of breeding prolongs the reproductive period (Bears et al. 2009). We propose these differences in productivity to be linked to differences in habitat structure and thus suitability between grassland patch groups. First, a Meadow Pipit population in Poland showed higher nest survival at moist and hidden microrelief structures Halupka (1998b). Consistent with this finding, the Hornisgrinde raised bog provides highly structured microrelief coupled with short and sparse vegetation and close-cropped grass areas that provide well-protected nest sites and the required accessible foraging habitat (Hölzinger and Ebenhöh 1999; Vandenberghe et al. 2009) throughout the breeding season. This contrasts to the other two patch groups, which are characterised by comparably little microrelief and rather dense and homogenous ground vegetation cover that is less penetrable for foraging, in particular in the late breeding season. Second, earlier work found that edge effects can affect nest survival (Vetter et al. 2013). The Hornisgrinde patch group contains the largest grassland patch in the study area, thus showing the smallest possible edge effects among all our study sites.
Conservation implications
Based on our findings above, we propose to expand low-intensity permanent cattle grazing to break up dense ground vegetation during the late breeding period (Bunzel-Drüke et al. 2019) and thus help to create more suitable structures like well-protected nest sites, accessible foraging habitat, and thus ultimately for successful first and second broods. Such predictable, permanent, and low intensity grazing regimes are not expected to result in relevant nest losses from trampling (Beintema and Muskens 1987, own data), contrary to rotational or paddock grazing systems, intense sheep grazing, or mowing that all go along with substantial nest losses in Meadow Pipit (own data, Pavel 2004) and other ground nesting birds (e.g., Handschuh and Klamm 2022). Since nestlings fledge by mid-August and require another approx. 2 weeks until showing full escape flights, intense grazing or mowing should start no earlier than late August (Glutz von Blotzheim and Bauer 1985). Management measures should further aim at restoring open grassland habitats lost to shrub succession. Besides space for more territories, larger grassland patches might also increase reproductive success due to more possibilities for foraging and nesting and reduced edge effects.
Data availability
All data generated or analyzed during this study are included in the supplementary information files.
References
Anthes N, Boschert M, Daniels-Trautner J (2017) Verbreitung und Bestandsentwicklung der Grauammer Emberiza calandra in Baden-Württemberg. Ornithol Jh Bad-Württ 33:27–44
Barras AG, Blache S, Schaub M, Arlettaz R (2021) Variation in demography and life-history strategies across the range of a declining mountain bird species. Front Ecol Evol 9:780706. https://doi.org/10.3389/fevo.2021.780706
Bauer HG, Heine G, Schmitz D, Segelbacher G, Werner S (2019) Starke Bestandsveränderungen der Brutvogelwelt des Bodenseegebietes – Ergebnisse aus vier flächendeckenden Brutvogelkartierungen in drei Jahrzehnten. Vogelwelt 139:3–29
Bears H, Martin K, White GC (2009) Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. J Anim Ecol 78:365–375. https://doi.org/10.1111/j.1365-2656.2008.01491.x
Beintema AJ, Muskens GJDM (1987) Nesting success of birds breeding in Dutch agricultural grasslands. J Appl Ecol 24:743–758. https://doi.org/10.2307/2403978
Berner D, Amrhein V (2022) Why and how we should join the shift from significance testing to estimation. J Evol Biol 35:777–787. https://doi.org/10.1111/jeb.14009
Bird Life International (2021) Anthus pratensis. The IUCN red list of threatened species 2021: e.T22718556A154480081. https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T22718 556 A154480081.en. Accessed 20 Dec 2022
Boatman ND, Brickle NW, Hart JD, Milsom TP, Morris AJ, Murray AWA, Robertson PA (2004) Evidence for the indirect effects of pesticides on farmland birds. Ibis 146:131–143. https://doi.org/10.1111/j.1474-919X.2004.00347.x
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal 9(2):378–400. https://doi.org/10.3929/ethz-b-000240890
Bunzel-Drüke M, Reisinger E, Böhm C, Buse J, Dalbeck L, Ellwanger G et al (2019) Naturnahe Beweidung und NATURA 2000, 2nd edn. Arbeitsgemeinschaft Biologischer Umweltschutz, Bad Sassendorf
Burns F, Eaton MA, Burfield IJ, Klvaňová A, Šilarová E, Staneva A, Gregory RD (2021) Abundance decline in the avifauna of the European Union reveals cross-continental similarities in bio-diversity change. Ecol Evol 11(23):16647–16660. https://doi.org/10.1002/ece3.8282
Constant P, Eybert MC (1980) Données sur la biologie de la reproduction du pipit farlouse, Anthus pratensis L., dans les landes bretonnes. Oiseaux 35:349–360
Coulson JC (1956) Mortality and egg production of the Meadow Pipit with special reference to altitude. Bird Study 3:119–132
Davies SJJF (1958) The breeding of the Meadow Pipit in Swedish Lapland. Bird Study 5:184–191. https://doi.org/10.1080/00063655809475919
Donald PF, Evans AD, Muirhead LB, Buckingham DL, Kirby WB, Schmitt SIA (2002) Survival rates, causes of failure and productivity of Skylark Alauda arvensis nests in lowland farmland. Ibis 144:652–664. https://doi.org/10.1046/j.1474-919X.2002.00101.x
Donald PF, Sanderson FJ, Burfield IJ, van Bommel FPJ (2006) Further evidence of continentwide impacts of agricultural intensification on European farmland birds, 1990–2000. Agric Ecosyst Environ 116:189–196. https://doi.org/10.1016/j.agee.2006.02.007
Einstein J, Harry I, Kramer M (2021) Bestandsentwicklung und Verbreitung des Braunkehlchens (Saxicola rubetra) in Baden-Württemberg seit 1950. Ornithol Jh Bad-Württ 37:7–19
European Bird Census Council (2022) Pan-European common bird monitoring scheme. https://pecbms.info/trends-and-indicators/species-trends/species/anthus-pratensis/. Accessed 3 Nov 2023
Förschler MI, Borras A, Cabrera J, Cabrera T, Senar JC (2005) Inter-locality variation in reproductive success of the Citril finch Serinus citrinella. J Ornithol 146:137–140. https://doi.org/10.1007/s10336-005-0072-y
Förschler MI, Anger F, del Val E, Aichele D, Dreiser C (2016a) Zur aktuellen und historischen Bestandssituation des Wiesenpiepers Anthus pratensis im Nordschwarzwald. Ornithol Jh Bad-Württ 32:45–51
Förschler MI, Richter C, Gamio T (2016b) Grinden – waldfreie Bergheiden im Nationalpark Schwarzwald. NaturschutzInfo 2(2016):28–31
Frey M (1989) Brutbiologie des Hänflings Carduelis cannabina unter den Einflüssen des Gebirgsklimas. Ornithol Beob 86:265–289
Gedeon K, Grüneberg C, Mitschke A, Sudfeldt C, Eickhorst W, Fischer S et al (2014) Atlas Deutscher Brutvogelarten. Stiftung Vogelmonitoring und Dachverband Deutscher Avifaunisten, Münster
Glutz von Blotzheim UN, Bauer KM (1985) Handbuch der Vögel Mitteleuropas. Passeriformes (1 Teil), 2nd edn. AULA-Verlag, Wiesbaden
Halsey LG, Curran-Everett D, Vowler SL, Drummond GB (2015) The fickle P value generates irreproducible results. Nat Methods 12:179–185. https://doi.org/10.1038/nmeth.3288
Halupka K (1998a) Nest predation in Meadow Pipits Anthus pratensis nesting in natural conditions. Ornis Fenn 75:139–143
Halupka K (1998b) Nest-site selection and nest predation in meadow pipits. Folia Zool 47:29–37
Handschuh M, Klamm A (2022) Nationally important population of the Corn Bunting Emberiza calandra in the Hainich National Park. Anzeiger Des Vereins Thüringer Ornithologen 10:43–78
Hölzinger J, Ebenhöh H (1999) Anthus pratensis (Linnaeus, 1758) Wiesenpieper. Seite 146–155 in: Hölzinger, J. (Herausgeber): Die Vögel Baden-Württembergs. Band 3.1, Singvögel 1. Ulmer, Stuttgart. Jochen Hölzinger
Hötker H, Sudfeldt C (1982) Untersuchungen zur Brutbiologie des Wiesenpiepers (Anthus pratensis). J Ornithol 123:183–201. https://doi.org/10.1007/BF01645057
Johnson DH (1979) Estimating nest success: the Mayfield method and an alternative. Auk 96:651–661. https://doi.org/10.1093/auk/96.4.651
Kamp J, Frank C, Trautmann S, Busch M, Dröschmeister R, Flade M et al (2020) Population trends of common breeding birds in Germany 1990–2018. J Ornithol 162:1–15. https://doi.org/10.1007/s10336-020-01830-4
Keller V, Herrando S, Voříšek P, Franch M, Kipson M, Milanesi P et al (2020) European breeding bird atlas 2: distribution, abundance and change. European Bird Census Council & Lynx Edicions, Barcelona
Kramer M, Bauer HG, Bindrich F, Einstein J, Mahler U (2022) Red List of Breeding Birds of Baden-Württemberg. 7th edition, as of 31 December 2019. – Naturschutz-Praxis Artenschutz 11. LUBW Landesanstalt für Umwelt Baden-Württemberg, Karlsruhe. www.lubw.baden-wuerttemberg.de
Laake J, Rexstad E (2008) RMark – an alternative approach to building linear models in MARK. http://www.phidot.org/software/mark/docs/book/pdf/app_3.pdf. Accessed 11 Aug 2023
Malm LE, Pearce-Higgins JW, Littlewood NA, Karley AJ, Karaszewska E, Jaques R et al (2020) Livestock grazing impacts components of the breeding productivity of a common upland insectivorous passerine: results from a long-term experiment. J Appl Ecol 57:1514–1523. https://doi.org/10.1111/1365-2664.13647
Menke W (2015) Die Entwicklung des Brutvogelbestands im Elisabeth-Außengroden (Gemeinde Wangerland, Kreis Friesland). Nachrichten Des Marschenrates 52:57–72
Newton I (2004) The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146:579–600. https://doi.org/10.1111/j.1474-919X.2004.00375.x
Olmeda C, Šefferová V, Underwood E, Millan L, Gil T, Naumann S (2020) EU Action plan to maintain and restore to favourable conservation status the habitat type 4030 European dry heaths. European Commission, pp 1–116
Pavel V (2004) The impact of grazing animals on nesting success of grassland passerines in farmland and natural habitats: a field experiment. Folia Zool 53:171–178
PECBMS (2023) Common farmland birds indicator. https://pecbms.info/trends-and-indicators/indicators/indicators/E_C_Fa/. Last Accessed 10 Jan 2023
Pedroli JC (1978) Breeding success of the Meadow Pipit Anthus pratensis in the Swiss Jura. Ornis Scand 9:168–171. https://doi.org/10.2307/3675878
Plard F, Bruns HA, Cimiotti DV, Helmecke A, Hötker H, Jeromin H et al (2020) Low productivity and unsuitable management drive the decline of central European lapwing populations. Anim Conserv 23:286–296. https://doi.org/10.1111/acv.12540
Rose LN (1982) Breeding ecology of British pipits and their cuckoo parasite. Bird Study 29:27–40. https://doi.org/10.1080/00063658209476735
Rotella JJ, Dinsmore SJ, Shaffer TL (2004) Modelling nest-survival data: a comparison of recently developed methods that can be implemented in MARK and SAS. Anim Biodivers Conserv 27:187–205
Santon M, Korner-Nievergelt F, Michiels NK, Anthes N (2023) A versatile workflow for linear modelling in R. Front Ecol Evol 11:1065273. https://doi.org/10.3389/fevo.2023.1065273
Seidt M, Geißler-Strobel S, Kramer M, Kratzer R, Straub F, Anthes N (2017) Bestandsentwicklung und Grundlagen für den Schutz des Rebhuhns Perdix perdix im Landkreis Tübingen. Ornithol Jh Bad-Württ 33:3–12
Seneviratne SI, Nicholls N, Easterling D, Goodess CM, Kanae S, Kossin J, Luo Y, Marengo J, McInnes K, Rahimi M, Reichstein M, Sorteberg A, Vera C, Zhang X (2012) Changes in climate extremes and their impacts on the natural physical environment. In: Field CB, Barros V, Stocke TF et al (eds) Managing the risks of extreme events and disasters to advance climate change adaptation. Cambridge University Press, Cambridge and New York, pp 109–230. https://doi.org/10.7916/d8-6nbt-s431
Südbeck P, Andretzke H, Fischer S, Gedeon K, Schikore T, Schröder K, Sudfeldt C (2005) Methodenstandards zur Erfassung der Brutvögel Deutschlands. Selbstverlag, Radolfzell
Vandenberghe C, Prior G, Littlewood NA, Brooker R, Pakeman R (2009) Influence of livestock grazing on meadow pipit foraging behaviour in upland grassland. Basic Appl Ecol 10:662–670. https://doi.org/10.1016/j.baae.2009.03.009
Vetter D, Rücker G, Storch I (2013) A meta-analysis of tropical forest edge effects on bird nest predation risk: edge effects in avian nest predation. Biol Conserv 159:382–395. https://doi.org/10.1016/j.biocon.2012.12.023
Wetterdienst D (2022) Klimadaten Deutschland, Station Freudenstadt. https://www.dwd.de/DE/leistungen/klimadatendeutschland/klimadatendeutschland.html. Accessed 20 Dec 2022
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139. https://doi.org/10.1080/00063659909477239
Zeder J, Fischer EM (2020) Observed extreme precipitation trends and scaling in Central Europe. Weather Clim Extremes 29:100266. https://doi.org/10.1016/j.wace.2020.100266
Acknowledgements
We thank Johannes Kamp for constructive comments on earlier versions of the manuscript. We acknowledge support from the Open Access Publishing Fund of the University of Tübingen.
Funding
Open Access funding enabled and organized by Projekt DEAL. FA was temporarily funded by the Landesgraduiertenförderung Baden-Württemberg, Germany.
Author information
Authors and Affiliations
Contributions
FA: conceptualization (lead); data curation (lead); investigation (lead); methodology (lead); project administration (lead); formal analysis (equal); software (support); writing—original draft preparation (lead); funding acquisition (lead). MIF: conceptualization (support); investigation (support), project administration (support); resources (lead); writing—review & editing (support). NA: conceptualization (support); formal analysis (equal); software (lead); methodology (support); writing—review & editing (lead).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Field work in this study was conducted under permits for the years 2020–2022 issued by the Black Forest National Park and the regional conservation authorities (Regierungspräsidium Freiburg and Karlsruhe, Az 84-8675.12 and Az 55-8841.03; 8853.17). Meadow Pipits in the study area are accustomed to human presence and animal welfare had priority. Nest visits were made as short as possible and none of the parents deserted after nest visits.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anger, F., Förschler, M.I. & Anthes, N. Variation in reproductive success in a fragmented Meadow Pipit population: a role for vegetation succession?. J Ornithol 165, 369–379 (2024). https://doi.org/10.1007/s10336-023-02121-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02121-4