Abstract
Imprinting, the process of forming lasting social bonds with early encountered stimuli, has been the subject of extensive research. However, there is still a need to systematically study the optimal methods for displaying imprinting stimuli in laboratory settings. This study aimed to investigate the effectiveness of different virtual presentation methods for imprinting stimuli and their impact on the memory of chicks. In the first experiment, we examined the attractiveness of various flickering frequencies, comparing them to static and translatory motion stimuli. The results revealed that flickering frequencies between 0.5 and 5 Hz were particularly appealing to newly hatched chicks, while higher frequencies (10–40 Hz) were less effective. We observed no significant differences in attractiveness between low flickering frequencies, moving stimuli, and static stimuli. In the second experiment, the focus shifted to the development of imprinting preference and memory. We found no significant difference in terms of preference for the imprinting stimulus between chicks imprinted with translatory motion or static stimuli. However, imprinting with flickering stimuli produced varied preferences. Chicks imprinted with a 2 Hz flickering stimulus exhibited a preference for the imprinting stimulus, albeit weaker than those imprinted with moving stimuli, while chicks imprinted with a 1 Hz flickering stimulus did not show a preference. These findings suggest that imprinting with flickering frequencies is not as effective as imprinting with moving stimuli and, to a lesser extent, static stimuli. Future studies should aim to determine the most optimal low frequencies within the 0.5–5 Hz range and explore different motion types. Overall, this research enhances our understanding of imprinting and provides valuable insights into virtual stimulation methods, thus informing the design of experiments in virtual environments.
Zusammenfassung
Flackernde Reize bei der Prägung
Prägung, der Prozess der Bildung dauerhafter sozialer Bindungen durch frühe Reize, ist Gegenstand umfangreicher Forschungsarbeiten. Es besteht jedoch immer noch Bedarf an einer systematischen Untersuchung der optimalen Methoden für die Präsentation von Prägungsreizen in Laborsituationen. Ziel dieser Studie war es, die Wirksamkeit verschiedener virtueller Präsentationsmethoden für Prägungsreize und ihre Auswirkungen auf das Gedächtnis von Küken zu untersuchen. Im ersten Experiment untersuchten wir die Attraktivität verschiedener Flackerfrequenzen und verglichen sie mit statischen und translatorischen Bewegungsreizen. Die Ergebnisse zeigten, dass Flackerfrequenzen zwischen 0,5 und 5 Hz für frisch geschlüpfte Küken besonders attraktiv waren, während höhere Frequenzen (10 bis 40 Hz) weniger wirksam waren. Es wurden keine signifikanten Unterschiede in der Attraktivität zwischen den niedrigen Flackerfrequenzen, den sich bewegenden Reizen und den statischen Reizen festgestellt. Im zweiten Experiment lag der Schwerpunkt auf der Entwicklung der Prägepräferenz und des Gedächtnisses. Wir fanden keinen signifikanten Unterschied in der Präferenz für den Prägungsreiz zwischen Küken, die mit translatorischen Bewegungsreizen oder statischen Reizen geprägt wurden. Küken, die mit einem 2-Hz-Flackerstimulus geprägt wurden, zeigten eine Präferenz für den prägenden Stimulus, wenn auch schwächer als Küken, die mit bewegten Stimuli geprägt wurden, während Küken, die mit einem 1-Hz-Flackerstimulus geprägt wurden, keine Präferenz zeigten. Diese Ergebnisse deuten darauf hin, dass das Einprägen mit flackernden Frequenzen nicht so effektiv ist wie das Einprägen mit sich bewegenden Reizen und, in geringerem Maße, mit statischen Reizen. Künftige Studien sollten darauf abzielen, die optimalen niedrigen Frequenzen im Bereich von 0,5 bis 5 Hz zu bestimmen und verschiedene Bewegungsarten zu untersuchen. Insgesamt verbessert diese Forschung unser Verständnis von Prägung und liefert wertvolle Einblicke in virtuelle Stimulationsmethoden und damit Informationen für die Gestaltung von Experimenten in virtuellen Umgebungen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Konrad Lorenz introduced the concept of imprinting in his seminal work, "Der Kumpan in der Umwelt des Vogels", published in The Journal of Ornithology in 1935. Imprinting is a process allowing precocial animals, especially nidifugous birds, to form a lasting social bond with a conspicuous stimulus encountered at an early age (Vallortigara and Versace 2018). In his seminal work, Lorenz had already noted that imprinting was closely associated with the bird's "inborn perceptory patterns", which serve as innate biases guiding the young birds in selecting proper stimuli.
Building on Lorenz's seminal work and taking advantage of the domestic chick as a model organism, ethologists began to experimentally investigate specific aspects of imprinting, such as the timing, duration, and characteristics of the stimuli that elicit and guide this process (Ramsay and Hess 1954). Just after hatching, chicks were exposed to a plethora of stimuli while scientists focused on two measures: the initial responses and approach/following behaviors of the chicks toward a stimulus, and the ability of the chicks to discriminate objects they have been exposed to (also termed ‘imprinting stimuli’) from novel ones (Andrew 1991; Bateson 1964; Bolhuis 1991). While the extent to which chicks initially approach and follow one stimulus has often been used to assess the effectiveness of a stimulus in eliciting imprinting, the extent to which chicks discriminate their imprinting stimulus from novel ones has commonly been used to evaluate the strength and development of imprinting and its memory. Using these measurements, it has been shown that imprinting must occur in the first days following hatching, typically up to day three in chicks (Yamaguchi et al. 2012) and that 14 h of exposure to a predisposed stimulus are sufficient for domestic chicks to develop a strong and steady preference for it (Lemaire et al. 2021). Additionally, certain features, such as color (red, orange, and blue), structures (face-like stimuli or head regions), and motion patterns (biological motion), are more likely to attract chicks' attention as well as facilitate future imprinting, confirming Lorenz’s original observations (Bolhuis 1991; Lemaire and Vallortigara 2023; Miura et al. 2019; Regolin et al. 2000; Rosa-Salva et al. 2015, 2021; Vallortigara 2021).
Nowadays, imprinting has become a valuable tool for investigating cognition and the onset of social behaviors in the laboratory (Di Giorgio et al. 2017; Rogers 2014; Rose 2000, 2003; Solomonia and McCabe 2015; Versace and Vallortigara 2015). However, certain aspects related to the optimal methods for leveraging the imprinting phenomenon have yet to be fully elucidated. While previous studies suggested that real moving stimuli are more effective than stationary ones in eliciting imprinting (Ten Cate 1986, 1989), other research suggested that flickering stimuli—i.e., stimuli appearing and disappearing at a specific rate—are as effective as motion stimuli (James 1959, 1960; Pumphrey 1948). Over the years, different flickering rates have been studied using flickering lights (Gottlieb and Simner 1969; Simner 1973), and it has been found that lights flickering at around 4 Hz were more effective at eliciting approach while lights flickering at higher frequencies (such as 12 and 24 Hz) were aversive. Now that the study of imprinting has moved from exposure to real stimuli to virtual ones, the most effective procedures for displaying stimuli on video screens for imprinting purposes require further investigation.
The present study aims to elucidate the effectiveness of different virtual stimuli presentation methods in an imprinting context and assess how these influence the imprinting memory of domestic chicks. Our investigation is based on the use of an automated setup previously developed (Lemaire et al. 2021; Zanon et al. 2021) and utilizes well-established imprinting procedure and stimuli, for which we know chicks’ imprinting preferences and naive biases (Lemaire et al. 2021). In a first experiment, we evaluated the attractiveness of a stimulus to newly hatched chicks by manipulating its dynamical presentation on screens. Initially, we assessed the attractiveness of various flickering frequencies to find the most attractive ones below the chicks’ critical flicker fusion frequency (Lisney et al. 2011). Subsequently, we selected the most appealing frequencies and compared them to other presentation methods (translatory motion and stationary display) that have been reported to have varying levels of attractiveness and that could influence imprinting. In the second experiment, we investigated whether different presentation methods of an imprinting stimulus influence chicks’ memory and development of the imprinting memory. To achieve this, we exposed chicks to the same stimulus as in the first experiment, using the different motion methods (stationary, translatory motion, and flickering) and subsequently assessed the chicks' preference for their imprinting stimulus in a dual-choice test, contrasting it with a novel stimulus.
Methods
Subjects
We used 272 White Leghorn chicks of the Ross 308 strain (200 in Experiment 1 and 72 in Experiment 2). Eggs were obtained from a local hatchery (Azienda Agricola Crescenti, Brescia) and incubated in our laboratory at the University of Trento at 37.7 °C and 40% humidity. To prevent tactile and visual experiences for the chicks, hatching occurred in individual black boxes located within a darkened hatching chamber maintained at 37.7 °C and 60% humidity. All procedures received approval from the Ethical Committee of the University of Trento and the Italian Ministry of Health (permit number 324/2022-PR).
Apparatus
The experimental apparatus consisted of a rectangular arena (60 cm × 90 cm × 90 cm; see Fig. 1a) equipped with high-frequency screens positioned on the shortest walls and used to display stimuli (for additional details, refer to Zanon et al. 2021). The animal behaviors were recorded using a Microsoft LifeCam mounted 105 cm above the apparatus. Food and water were available ad libitum in the apparatus center. Nine apparatuses were used concurrently.
Stimuli and presentation
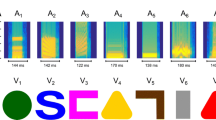
We employed 3D stimuli (a blue -hex: 2EBAFF- cube and a green -hex: 30B619- hourglass) previously used in a study investigating imprinting preferences over time (Lemaire et al. 2021). The stimuli were displayed as 2D images on high-frequency screens with a refresh rate of 120 Hz. Using 'ImprintSchedule', an open-source program (described in Zanon et al. 2021), we manipulated the methods used to display our stimuli. We utilized three distinct motion paradigms: flickering (with 9 levels tested in Experiment 1: 0.5, 1, 2, 3, 4, 5, 10, 20, and 40 Hz; and 2 levels tested in Experiment 2: 1 and 2 Hz), translatory motion (horizontally moving back and forth across the screen length in 9 s), or stationary (static image at the screen center) (see Fig. 1b, c). In the flickering stimulation type, stimuli were presented with equal durations of appearance and disappearance, ensuring a consistent timing pattern across all tested frequencies.
Procedure
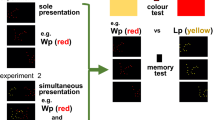
In both experiments, immediately after hatching, chicks were briefly sexed by examining their feathers under dim light and then placed in the center of their individual apparatus where they remained for the entire duration of the experiments. Experiment 1 consisted of a 14-h exposure period during which chicks were exposed to the blue cube described earlier (imprinting phase). These 14 h were divided into 14 sessions of 59 min, interrupted by 1-min intervals of black screens (Fig. 1b). The appearance of the stimuli on the screens was counterbalanced across sessions. According to Lemaire et al. (2021), this procedure is known to induce strong and stable imprinting preferences with the blue cube used here.
Experiment 2 started with the same procedure as Experiment 1 (as shown in Fig. 1c), but the chicks remained in their apparatus for the entire night (10 h with empty black screens) to be tested the following day. During the test phase, which lasted 14 h, the chicks were exposed to their imprinting stimulus (blue cube) as well as a novel stimulus (green hourglass). The 14 h of testing were divided into 25 sessions of 30 min each, with each session interrupted by a 5-min black screen interval. Before the first test session, the chicks were gently relocated to the center of their apparatus to avoid any positional bias. As for the imprinting sessions, the appearance of the stimuli on the screens was counterbalanced across testing sessions.
Data analysis
In both experiments, we used DeepLabCut (Nath et al. 2019) to track the location of the chicks within the cage, and we assessed their preference for a stimulus by measuring the time spent close to it. An area within 30 cm from the screen was considered as a choice for the relative stimulus.
During the imprinting phase of both experiments, we analyzed the time spent in the closest zone to the stimulus (values ranging from 0 to 14 h) across different stimulation types (9 in the primary analysis of experiment 1 and 4 levels in the secondary analysis of experiment 1: [0.5, 1, 2, 3, 4, 5, 10, 20, 40 Hz] and [stationary, translatory motion, 1 and 2 Hz] respectively); 4 levels in experiment 2: stationary, translatory motion, 1 and 2 Hz) and sex (2 levels). The data was log transformed to meet parametric assumptions. In case the assumptions were met, we performed analyses of variance (ANOVA), followed by t-tests to investigate the significant effects. When the parametric assumptions were not met (even after data transformation), we performed a permutation test using the aovp function (lmPerm package) as a non-parametric equivalent of the ANOVA followed by t-tests to investigate the significant effects.
During the test phase of experiment 2, we analyzed the chicks’ preference for the imprinting stimulus compared to a novel stimulus across different stimulation types (4 levels: stationary, translatory motion, and 2 different flickering frequencies [1 and 2 Hz]) and sex (2 levels). We calculated the preference for the imprinting stimulus (PI) using the following formula:
where timprinting stimulus is the time spent close to the imprinting stimulus and ttotal is the sum of time spent close to both stimuli.
A score of 50% indicates no preference, a score above 50% indicates a preference for the imprinting stimulus and a score below 50% indicates a preference for the unfamiliar stimulus. As the parametric assumptions were not met, we performed a non-parametric analysis for longitudinal data (nparLD package) that included the different stimulation types, sex and time (25 test sessions) as factors. Finally, for the overall and longitudinal analyses, we performed t-tests to investigate the chicks' preference for the imprinting stimulus against the chance level (index of preference at 50%) and between groups. In addition, we performed Pearson tests to investigate whether the time spent by the chicks close to their imprinting stimulus during the imprinting phase was correlated with the chicks’ preference for their imprinting stimulus during the overall and first session of the testing phase.
Results
Experiment 1
The data are shown in Fig. 2. The analysis revealed a significant effect of frequency (F(8,153) = 12.64, p < 0.001), but no effect of sex (F(1,153) = 3.14, p = 0.08) and no interaction effect (F(8,153) = 1.11, p = 0.36). Chicks spent more time near the stimulus with lower frequencies (0.5, 1, 2, 3, 4, 5 Hz) compared to higher frequencies (10, 20, 40 Hz; see supplementary Table S1 for pairwise comparisons and descriptive statistics). Among the lower frequencies, 2 and 4 Hz seemed most effective in attracting chicks to the stimulus (2 Hz: mean = 11.56 h, 95% CI = 10.20–12.92; 4 Hz: mean = 11.78 h, 95% CI = 10.45–13.11), while 0.5, 1, 3, and 5 Hz elicited slightly lower responses (0.5 Hz: mean = 11.11, 95% CI = 10.09–12.13; 1 Hz: mean = 10.24, 95% CI = 8.81–11.68; 3 Hz: mean = 11.17, 95% CI = 10.19–12.15; 5 Hz: mean = 10.09, 95% CI = 8.45–11.74). Among the higher frequencies, 10 Hz (mean = 7.63, 95%CI = 5.82–9.43) was more effective than 20 and 40 Hz (20 Hz: mean = 6.71, 95% CI = 5.34–8.10; 40 Hz: mean = 5.61, 95% CI = 4.35–6.87), but none of the pair comparisons reaches significance (see Table S1 for descriptive statistics).
Time spent close to the imprinting stimulus across the 14 imprinting hours, displayed at different frequencies (statistics are reported in Table S1). Data points represent the individual scores of males in purple and females in orange
Flickering stimuli have not often been used to imprint chicks on virtual stimuli in the laboratory. To further explore the effectiveness of the lower flickering frequencies, we selected two of the flickering frequencies producing strong attraction responses, and compared them with two common stimulation types used in laboratory settings: translatory motion and stationary display. Among the lowest frequencies, we selected 2 Hz, which visually elicited one of the strongest responses with the smallest interquartile range and whiskers and 1 Hz which visually elicited one of the smallest responses with the largest interquartile range and whiskers. The results are shown in Fig. 3. Our ANOVA revealed no significant effect of stimulation type (F(3,67) = 0.91, p = 0.44), sex (F(1,67) = 1.34, p = 0.24), or interaction (F(3,67) = 2.28, p = 0.087). Static (mean = 10.69, 95% CI = 9.43–11.95) and translatory (mean = 10.38, 95% CI = 8.96–11.81) stimuli showed similar results to the flickering ones (1 Hz: mean = 10.24, 95% CI = 8.81–11.68; 2 Hz: mean = 11.56, 95% CI = 10.20–12.92).
Experiment 2
In the second experiment, we examined whether similar levels of attractiveness would result in the development of different filial imprinting preferences and memory. We replicated the imprinting phase of Experiment 1 including two low flickering frequencies (1 and 2 Hz) translatory and static stimulations. Following imprinting, we then tested the chicks' preference for their imprinting stimulus in a repeated dual-choice task with a novel stimulus, following the procedure of Lemaire et al. (2021).
Imprinting phase
During the imprinting phase, when chicks were exposed to only one stimulus, we found no significant differences between the stimulation types (flickering 1 Hz, flickering 2 Hz, translatory motion, and stationary; F(3,64) = 0.52, p = 0.67), sex (F(1, 64) = 0.01, p = 0.92), or interactions (F(3,64) = 0.36, p = 0.78). The data is shown in Fig. 4. All chicks showed similar attraction levels to the displayed stimulus (static: mean = 11.85, 95% CI = 11.02–12.67; translatory: mean = 11.32, 95% CI = 10.56–12.07; 1 Hz: mean = 11.47, 95% CI = 10.42–12.53; 2 Hz: mean = 11.56, 95% CI = 10.51–12.60), regardless of the stimulation type, replicating the results from the first experiment. Interestingly, the more nuanced response observed at 1 Hz was no longer visually perceived, suggesting that the distribution observed within the lowest frequencies in the first experiment was likely due to chance induced by the relatively small sample size.
Test phase
During the repeated exposure (25 sessions of 30 min) to the imprinting vs. unfamiliar stimulus, we found a significant effect of stimulation type (statistic(2.57) = 4.26, p < 0.01) but no effect of session (statistic(12.82) = 1.41, p = 0.15), sex (statistic(1) = 1.12, p = 0.29), or interactions (see statistics for interactions). The data is shown in Fig. 5. Overall, all stimulation types showed a significant preference for their imprinting stimulus (static: t(17) = 3.73, p value < 0.01, Cohen’s d = 0.88, mean = 64.92, 95% CI = 56.49–73.36; translatory: t(17) = 5.86, p value < 0.001, Cohen’s d = 1.38, mean = 67.97, 95% CI = 61.50–74.44; flickering [1 Hz]: t(17) = 2.34, p value < 0.05, Cohen’s d = 0.55, mean = 55.60, 95% CI = 50.54–60.66; flickering [2 Hz]: t(17) = 3.94, p value < 0.01, Cohen’s d = 0.93, mean = 59.89, 95% CI = 54.57–65.19). However, the preference strength varied between stimulation types. Chicks exposed to translatory stimuli had a stronger preference for their imprinting stimulus than chicks exposed to flickering stimuli (translatory vs. flickering [1 Hz]: t(32.13) = 3.18, p value < 0.01, Cohen’s d = 1.06; translatory vs. flickering [2 Hz]: t(32.73) = 2.04, p value < 0.05, Cohen’s d = 0.68) but not than chicks exposed to static stimuli (t(31.86) = 0.61, p value = 0.55, Cohen’s d = 0.20). Chicks exposed to static stimuli tended to have a stronger preference for their imprinting stimulus compared to chicks exposed to 1 Hz (t(27.83) = 2.0, p value = 0.055, Cohen’s d = 0.67) but not 2 Hz flickering stimuli (t(28.61) = 1.07, p value = 0.30, Cohen’s d = 0.36). Differences in preference between chicks exposed to 1 or 2 Hz flickering stimuli were not significant (t(33.93) = − 1.23, p value = 0.23, Cohen’s d = 0.41).
Chicks’ preference for their imprinting stimulus across sessions (b) and overall (a) between different stimulation types. In a, data points represent individual scores of males in purple and females in orange and stars represent significance levels (p < 0.05, *; p < 0.01, **; p < 0.001, ***). In b, error bars represent SEM
Although the non-parametric longitudinal analysis did not reveal any interaction between session and stimulation types (due to the large number of testing sessions and an overall preference for chicks’ imprinting stimulus), it is worth noting that the initial preference (see session 1) visually appeared different between stimulation types. Indeed, in the first testing session (first 30 min after the night period following imprinting), chicks exposed to translatory stimuli seemed to have an initial preference for the imprinting stimulus (mean = 68.07, 95% CI = 46.80–89.34). Intriguingly, this initial preference was absent when chicks were exposed to static stimuli (mean = 49.57, 95% CI = 27.31–71.83) and seemed to reverse when exposed to flickering stimuli (1 Hz: mean = 30.84, 95% CI = 11.32–50.37; 2 Hz: mean = 34.38, 95% CI = 15.62–53.13). However, none of these visual tendencies reached significance, likely due to the low sample size for a non-repeated measurement design (static: t(17) = − 0.040, p value = 0.97, Cohen’s d = 0.0096; translatory: t(17) = 1.079, p value = 0.09, Cohen’s d = 0.42; flickering [1 Hz]: t(17) = − 2.07, p value = 0.054, Cohen’s d = 0.49; flickering [2 Hz]: t(17) = − 1.76, p value = 0.097, Cohen’s d = 0.41).
Importantly, the observed preference index during the overall testing period and the first testing session did not show a significant correlation with the time spent by the chicks close to their imprinting stimulus during imprinting. Specifically, Pearson's test on data from the overall testing period yielded r = 0.08, with a p value of 0.52. Likewise, Pearson's test on data from the first testing session yielded r = 0.01, with a p value of 0.96.
Discussion
This study aimed to assess the effectiveness of different methods, used to display stimuli on video screens, in capturing the attention of chicks in an imprinting context. We examined various flickering frequencies to determine the most effective ones in eliciting approach and maintaining chicks’ attention, and compared them to static and moving stimuli commonly used in laboratory settings.
Our findings align with Simner's experiment (1973), which demonstrated that flickering lights with frequencies ranging from 0.5 to 4 Hz were more effective in capturing chicks' attention compared to higher frequencies of 12 and 24 Hz. Consistent with Simner's hypothesis, we observed that chicks exposed to a stimulus flickering between 0.5 and 5 Hz showed higher attraction as they spent significantly more time near it compared to chicks exposed to the same stimulus flickering at 10, 20, or 40 Hz. The underlying reasons for this early attraction to lower flickering frequencies remain to be further investigated, with theories suggesting a link to systemic rhythms present during hatching in chicks (Gottlieb 1968; Simner and Kaplan 1977).
Interestingly, our results suggest that chicks exhibit a similar level of attraction toward a stimulus flickering at low frequency compared to a stimulus that is either moving or static on the screen. While the idea proposed by Menner (Pumphrey 1948) and James (1959, 1960), stating that flickering should be as attractive as moving stimuli, is supported by our initial experiment, the absence of a difference in attractiveness between flickering and moving stimuli compared to static stimuli is surprising and not in line with the existing literature (Ten Cate 1986, 1989) nor with Lorenz's original observation (1935). One possible explanation could be the salience of the stimulus used in our experiment, as we employed a conspicuous and effective blue stimulus known to elicit strong imprinting responses. It is plausible that small differences in attractiveness may not have been discernible solely through the chicks' approach and following behaviors. Another possibility is that the sample size used in our initial experiment was insufficient to demonstrate significant differences for some of the lowest frequencies tested. Our second experiment supplemented our investigation by examining chicks' ability to discriminate between their imprinting stimulus and a novel stimulus after one day of imprinting, thereby providing a more accurate representation of imprinting development and memory.
First, it should be noted that the time chicks spent near their imprinting stimulus during the imprinting phase of this second experiment was not a strong predictor of their preference index, both during the overall testing period and the first testing session. The absence of correlation enabled us to dissociate the two measures. In terms of imprinting preferences, our overall results indicated no difference between chicks exposed to translatory motion and those exposed to static stimuli. Both methods yielded a similar preference and imprinting strength for the imprinting stimulus, replicating findings from Lemaire et al. (2021). However, distinctions emerged when chicks were imprinted with a flickering stimulus. Both frequencies exhibited a lower preference for their imprinting stimulus compared to translatory and to a lower extent to static stimulations. While the absence of a difference in imprinting preference between chicks imprinted with moving or static stimuli requires further investigation, our results indicate that imprinting with flickering frequencies does not generate the same preference observed in chicks imprinted with moving stimuli. Additionally, not all low frequencies were equally effective in eliciting imprinting and establishing a strong memory. Further experiments with larger sample sizes are warranted to identify the most efficient lowest frequencies (ranging from 0.5 to 5 Hz) in eliciting and maintaining imprinting memory, as well as to explore the slight temporal differences that were visually observed in the time profile, but that could not be statistically analyzed (see Fig. 5b).
In conclusion, our investigation provides valuable insights into the types of stimulation that can be employed in studies making use of imprinting in virtual environments. While we demonstrated that low flickering frequencies (0.5–5 Hz) were as effective as translatory and static stimulation in capturing and maintaining chicks' attention, we also found that some of the lowest frequencies were less effective in eliciting and building a strong imprinting memory. Conversely, high frequencies (10–40 Hz) were not effective in capturing and sustaining chicks' attention. Further investigations are needed to explore whether frequencies higher than 40 Hz could match the attractiveness levels of the lowest frequencies and to understand the development of imprinting memory with high frequencies. It is worth noting that our current experimental setup limited us to a maximum frequency of 40 Hz due to computational stability constraints. However, our results imply that high frequencies are less attractive compared to lower ones. Moreover, while our focus was primarily on determining optimal flicker rates compared to a single motion type and speed, it would be interesting to extend similar procedures to test different motion types and velocities that elicit imprinting and examine their influence on the development of imprinting memory. These future studies would provide a more comprehensive understanding of the effects of various stimuli on imprinting and its cognitive and social aspects in neonatal animals. Overall, our work presents a standardized procedure for investigating imprinting and leveraging it to explore cognition and various aspects of social behavior in neonatal animals. By uncovering the effectiveness of different stimuli in capturing and maintaining chicks' attention, our study contributes to the growing body of knowledge on animal behavior and offers valuable insights for designing experiments in virtual environments.
Availability of data and material
All the reported materials are freely available at https://doi.org/10.6084/m9.figshare.23259689.
References
Andrew RJ (1991) Neural and behavioural plasticity: the use of the domestic chick as a model. Oxford University Press
Bateson PPG (1964) Relation between conspicuousness of stimuli and their effectiveness in the imprinting situation. J Comp Physiol Psychol 58:407–411. https://doi.org/10.1037/h0045376
Bolhuis JJ (1991) Mechanisms of avian imprinting: a review. Biol Rev 66:303–345. https://doi.org/10.1111/j.1469-185X.1991.tb01145.x
Di Giorgio E, Loveland JL, Mayer U, Rosa-Salva O, Versace E, Vallortigara G (2017) Filial responses as predisposed and learned preferences: early attachment in chicks and babies. Behav Brain Res 325:90–104. https://doi.org/10.1016/j.bbr.2016.09.018
Gottlieb G (1968) Prenatal behavior of birds. Q Rev Biol 43:148–174. https://doi.org/10.1086/405726
Gottlieb G, Simner ML (1969) Auditory versus visual flicker in directing the approach response of domestic chicks. J Comp Physiol Psychol 67:58–63. https://doi.org/10.1037/h0026668
James H (1959) Flicker: an unconditioned stimulus for imprinting. Canad J Psychol/rev Can Psychol 13:59–67. https://doi.org/10.1037/h0083767
James H (1960) Imprinting with visual flicker: evidence for a critical period. Canad J Psychol/rev Can Psychol 14:13–20. https://doi.org/10.1037/h0083178
Lemaire BS, Vallortigara G (2023) Life is in motion (through a chick’s eye). Anim Cogn 26:129–140. https://doi.org/10.1007/s10071-022-01703-8
Lemaire BS, Rucco D, Josserand M, Vallortigara G, Versace E (2021) Stability and individual variability of social attachment in imprinting. Sci Rep 11:7914. https://doi.org/10.1038/s41598-021-86989-3
Lisney TJ, Rubene D, Rózsa J, Løvlie H, Håstad O, Ödeen A (2011) Behavioural assessment of flicker fusion frequency in chicken Gallus gallus domesticus. Vis Res 51:1324–1332. https://doi.org/10.1016/j.visres.2011.04.009
Lorenz K (1935) Der Kumpan in der Umwelt des Vogels. J Ornithol 83:289–413. https://doi.org/10.1007/BF01905572
Miura M, Nishi D, Matsushima T (2019) Combined predisposed preferences for colour and biological motion make robust development of social attachment through imprinting. Anim Cogn. https://doi.org/10.1007/s10071-019-01327-5
Nath T, Mathis A, Chen AC, Patel A, Bethge M, Mathis MW (2019) Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat Protoc 14:2152–2176. https://doi.org/10.1038/s41596-019-0176-0
Pumphrey RJ (1948) The sense organs of birds. Ibis 90:171–199. https://doi.org/10.1111/j.1474-919X.1948.tb01686.x
Ramsay E, Hess E (1954) A laboratory approach to the study of imprinting. Wilson J Ornithol 66:196–206
Regolin L, Tommasi L, Vallortigara G (2000) Visual perception of biological motion in newly hatched chicks as revealed by an imprinting procedure. Anim Cogn 3:53–60. https://doi.org/10.1007/s100710050050
Rogers LJ (2014) Asymmetry of brain and behavior in animals: Its development, function, and human relevance. Genesis 52:555–571. https://doi.org/10.1002/dvg.22741
Rosa-Salva O, Mayer U, Vallortigara G (2015) Roots of a social brain: developmental models of emerging animacy-detection mechanisms. Neurosci Biobehav Rev 50:150–168. https://doi.org/10.1016/j.neubiorev.2014.12.015
Rosa-Salva O, Mayer U, Versace E, Hébert M, Lemaire BS, Vallortigara G (2021) Sensitive periods for social development: interactions between predisposed and learned mechanisms. Cognition 213:104552. https://doi.org/10.1016/j.cognition.2020.104552
Rose SPR (2000) God’s organism? The chick as a model system for memory studies. Learn Mem 7:1–17. https://doi.org/10.1101/lm.7.1.1
Rose SPR (2003) The making of memory: from molecule to mind. Vintage
Simner ML (1973) The development of visual flicker rate preference in the newly hatched chick. Dev Psychobiol 6:377–384. https://doi.org/10.1002/dev.420060411
Simner ML, Kaplan W (1977) The cardiac self-stimulation hypothesis and the chick’s differential attraction toward intermittent sound. Dev Psychobiol 10:177–186. https://doi.org/10.1002/dev.420100211
Solomonia RO, McCabe BJ (2015) Molecular mechanisms of memory in imprinting. Neurosci Biobehav Rev 50:56–69. https://doi.org/10.1016/j.neubiorev.2014.09.013
Ten Cate C (1986) Does behavior contingent stimulus movement enhance filial imprinting in Japanese quail? Dev Psychobiol 19:607–614. https://doi.org/10.1002/dev.420190611
Ten Cate C (1989) Stimulus movement, hen behaviour and filial imprinting in Japanese quail (Coturnix coturnix japonica). Ethology 82:287–306. https://doi.org/10.1111/j.1439-0310.1989.tb00509.x
Vallortigara G (2021) Born knowing. The origins of knowledge. MIT Press
Vallortigara G, Versace E (2018) Filial imprinting. Encyclopedia of animal cognition and behavior. Springer International Publishing, Cham, pp 1–4. https://doi.org/10.1007/978-3-319-47829-6_1989-1
Versace E, Vallortigara G (2015) Origins of Knowledge: insights from precocial species. Front Behav Neurosci. https://doi.org/10.3389/fnbeh.2015.00338
Yamaguchi S, Aoki N, Kitajima T, Iikubo E, Katagiri S, Matsushima T, Homma KJ (2012) Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat Commun 3:1081. https://doi.org/10.1038/ncomms2088
Zanon M, Lemaire BS, Vallortigara G (2021) Steps towards a computational ethology: an automatized, interactive setup to investigate filial imprinting and biological predispositions. Biol Cybern 115:575–584. https://doi.org/10.1007/s00422-021-00886-6
Funding
Open access funding provided by Università degli Studi di Trento within the CRUI-CARE Agreement. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement number 833504-SPANUMBRA to G.V.) and by Progetti di Rilevante Interesse Nazionale (PRIN 2017 ERC-SH4–A 2017PSRHPZ to G.V.)
Author information
Authors and Affiliations
Contributions
M.Z. and B.W. acquired the data. M.Z. and B.S.L. performed the analysis. G.V. supervised the project. B.S.L., M.Z., S.P. and G.V. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection 50 years anniversary of the Nobel Prize in Physiology or Medicine to Karl von Frisch, Konrad Lorenz and Niko Tinbergen in 1973.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lemaire, B.S., Zanon, M., Placì, S. et al. Flickering stimuli presentation in imprinting. J Ornithol 165, 39–48 (2024). https://doi.org/10.1007/s10336-023-02110-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02110-7