Abstract
The giant accipitrid Dynatoaetus gaffae gen. et sp. nov. is described from existing and newly collected material. Initial fossil remains were collected from Mairs Cave (Flinders Ranges, South Australia) in 1956 and 1969, and comprised a sternum, distal humerus and two ungual phalanges. A further 28 bones from this individual—including the neurocranium, vertebrae, furculum, and additional wing and leg bones, most of which were incomplete—were discovered at the site in 2021. This allowed identification of additional fossils from the same species in collections from Cooper Creek (Lake Eyre Basin, SA), Victoria Fossil Cave (Naracoorte, SA) and Wellington Caves (Wellington, NSW). Dynatoaetus has variable similarity across elements to those of living species in the Perninae, Gypaetinae, Circaetinae and Aegypiinae. Parsimony and Bayesian phylogenetic analyses of combined morphological and DNA data resolved it as the immediate sister-group to the Aegypiinae within the Circaetinae + Aegypiinae clade. The robust and eagle-like morphology of the lower hindlimbs suggest that the species was a predator, rather than a scavenger, and thus functionally similar to large circaetines such as the Philippine Eagle Pithecophaga jefferyi. Furthermore, this new species is the largest known bird of prey from Australia, much larger than the modern Wedge-Tailed Eagle Aquila audax. It is outsized in Australasia only by female Hieraaetus moorei (the extinct Haast’s Eagle from New Zealand). It is inferred to have been Australia’s top terrestrial avian predator during the Pleistocene, ranging from arid inland Australia to the more temperate coast, and likely became extinct around the time of the megafaunal mass extinction which peaked around 50 Ka. Its extinction in the late Pleistocene, along with the recently described scavenging vulture Cryptogyps lacertosus, marked a distinct decline in the diversity and function of Australia’s raptor guild.
Zusammenfassung
Ein Riesen-Greifvogel (Aves: Accipitridae) aus dem Pleistozän Südaustraliens
Hier beschreiben wir den riesigen Accipitriden Dynatoaetus gaffae gen. et sp. nov. anhand von bereits bekanntem sowie neu gesammeltem Material. Die ursprünglichen Fossilbelege wurden in den Jahren 1956 und 1969 in Mairs Cave (Flinderskette, Südaustralien) gesammelt und umfassen das Brustbein, den distalen Humerus sowie zwei Fußphalangen. 2021 fand man an derselben Stelle 28 weitere Knochen dieses Individuums, darunter das Neurokranium, Wirbel, das Gabelbein sowie weitere Flügel- und Beinknochen, von denen die meisten nur teilweise erhalten und unvollständig sind. Dies ermöglichte die Bestimmung weiterer Fossilien derselben Art in Sammlungen aus Cooper Creek (Eyre-Becken, Südaustralien), Victoria Fossil Cave (Naracoorte, Südaustralien) und den Wellington Caves (Wellington, Neusüdwales). Dynatoaetus weist verschiedene Ähnlichkeiten mit rezenten Arten aus den Gruppen der Perninae, Gypaetinae, Circaetinae und Aegypiinae auf. Phylogenetische Analysen (Parsimonie und Bayes'sche Statistik) einer Kombination aus morphologischen und DNS-Daten identifizierten das Taxon als unmittelbare Schwestergruppe der Aegypiinae innerhalb der (Circaetinae + Aegypiinae)-Klade. Die kräftige, adlerähnliche Morphologie der unteren Hinterextremitäten legt nahe, dass die Art eher ein Beutegreifer als ein Aasfresser war und daher funktional großen Circaetinen wie beispielsweise Pithecophaga jefferyi nahesteht. Darüber hinaus ist die Art der größte bekannte Greifvogel Australiens, bedeutend größer als der rezente Keilschwanzadler Aquila audax. Im australasiatischen Raum wird er an Größe nur noch durch weibliche Exemplare des ausgestorbenen Haastadlers Hieraaetus moorei aus Neuseeland übertroffen. Die Art war vermutlich Australiens Spitzenprädator unter den Landvögeln des Pleistozäns, mit einer Verbreitung vom ariden Binnenland bis hin zu den gemäßigteren Küstenregionen Australiens, und verschwand vermutlich ungefähr zur Zeit des Massenaussterbens großer Tierarten vor circa 50.000 Jahren. Sein Aussterben im späten Pleistozän, zusammen mit dem kürzlich beschriebenen aasfressenden Geier Cryptogyps lacertosus, kennzeichnet eine deutliche Abnahme in Vielfalt und Funktion der australischen Greifvogelgilde.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fossil record of Australian accipitrids
The earliest known Australian accipitrids (eagles, hawks, and Old World vultures) are the late Oligocene species Archaehierax sylvestris Mather et al. (2021) from the Namba Formation at Lake Pinpa in South Australia and Pengana robertbolesi Boles (1993) from Riversleigh in north-western Queensland. These species were both adapted for forest habitats, with A. sylvestris having a shortened wingspan for its size (Mather et al. 2021) and P. robertbolesi possessing highly flexible legs that would likely have been capable of reaching into tree hollows to pull out prey (Boles 1993).
The post-Oligocene fossil record for Accipitridae remains quite limited. One species is known from the late Miocene 11–9 Ma, Aquila bullockensis Gaff and Boles (2010), which was based on an isolated distal humerus and is currently the oldest species of Aquila known from Australia. An undescribed eagle species was recorded from the ~ 8 Ma Alcoota site (Worthy and Yates 2018).
The Plio-Pleistocene Australian fossil record currently has two accepted extinct species of Accipitridae: Cryptogyps lacertosus (de Vis 1905; see Mather et al. 2022) and Necrastur alacer de Vis (1892). Several other accipitrid species were described by de Vis in the late nineteenth and early twentieth century but reviews of the original fossil material have either synonymized them with living raptors or discovered them to be non-avian (Rich et al. 1982; van Tets and Rich 1990; Boles 2006). Additional accipitrid fossils have been recognised from multiple cave sites across the continent, including several undescribed taxa (Gaff 2002; Mather 2021).
Pleistocene fossil sites containing undescribed fossil accipitrids
Mairs cave
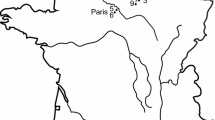
Mairs Cave is located in Buckalowie Gorge in the southern Flinders Ranges, South Australia (Fig. 1). It is approximately 400 m long, with the entrance chamber being 120 m long by 10 m wide (Kraehenbuehl et al. 1997; Treble et al. 2017). The floor of this entrance chamber slopes down towards the east and is covered in large piles of boulders in many places, among which fossils can be found. The sections of the chamber farthest from the entrance lack stratified sediment, and therein most of the larger vertebrate fossils have been found scattered amid the rocks. This presents a severe limitation in trying to determine the age of fossils, as there is little evidence to help estimate when they were originally deposited (Liddle et al. 2018).
Map of the entrance chamber of Mairs Cave (modified from original map produced by the Cave Exploration Group of South Australia [CEGSA], surveyed by R. T. Sexton 1958), with the location of the eagle fossil bones marked by the triangle symbol. The inset map of Australia shows sites where Dynatoaetus gaffae gen. et sp. nov. has been found
Several speleothems from Mairs Cave have been dated in various studies that provide some insight on the age of surface deposits. Gould-Whaley et al. (2021) dated subaqueous speleothem growth in a former pool in the entrance chamber to around 67 ka (kiloannum, thousand years) and 48 ka, and Treble et al. (2017) found evidence for wet phases during the last glacial maximum during the periods 23–18.9 ka and 18.9–15.8 ka in small surface speleothems. However, much older speleothem growth is known in the cave, and is the focus of ongoing research (J. Woodhead pers. comm. to THW).
A wide range of mammals, birds and reptiles are known from this site and a faunal list expanded as a result of collections made during this study, listed in SI.1. The extinct megafaunal component, e.g. the apex predator marsupial Thylacoleo carnifex, suggests the fauna in part dates to older than 40 ka (see Saltre et al. 2016; Hocknull et al. 2020).
Wellington Caves
Located in central-western New South Wales less than 10 km south of the town of Wellington (Fig. 1), the Wellington Caves are a source of fossiliferous deposits that are poorly dated but are suggested to span from the early Pliocene to the late Pleistocene (Dawson 1985, 1995; Fischer 1997; Dawson et al. 1999; Osborne 2001; Nipperess 2002; Bell 2004; Megirian et al. 2010). Multiple caves have produced fossils, including Cathedral Cave, Bone Cave, Phosphate Mine and Mitchell’s Cave (Dawson 1985; Osborne 1991, 1997). Many fossils from this site, particularly those from Mitchell’s Cave and Phosphate mine, have poor provenance data. These fossils, known as the “Old Collection”, were collected from the caves from the middle of the nineteenth century to the early twentieth century when the caves were under the management of the NSW Department of Mines (Dawson 1985). Among this “Old Collection” are several accipitrid fossils, some of which have been referred to Cryptogyps lacertosus (see Mather et al. 2022), while others have yet to be described.
Victoria Fossil Cave
Part of the World Heritage listed Naracoorte Caves in south-eastern South Australia, Victoria Fossil Cave is the source of multiple fossil deposits, most notably Fossil Chamber, Grant Hall and the Ossuaries (Reed 2003, 2006; Fraser and Wells 2006). The fossils within this cave date from the last 500 ka of the Pleistocene (Grün et al. 2001; Prideaux et al. 2007; Macken et al. 2011, 2013; Macken and Reed 2013). This cave is one of the most significant sites for Pleistocene vertebrate fossils in Australia and has provided a valuable record of mostly mammalian species, with some reptilian and avian elements also preserved.
Fossil Chamber, the source of some of the material described in this study, has fossil material dating between 500 and 213 ka (Ayliffe et al. 1998; Arnold et al. 2022). A recent study has indicated a potential upper (older) age limit of 600 ka for vertebrate fossils in the Naracoorte Cave Complex based on pollen and charcoal records (Weij et al. 2022). Accipitrid fossils are known from the very large and species-rich deposit in Fossil Chamber but are extremely rare, with only eight bones currently identified (see Mather 2021).
Cooper Creek
The Cooper Creek, which flows across south-western Queensland into north-eastern South Australia before ending at Kati Thanda-Lake Eyre, is part of the Lake Eyre Basin. Most fossil sites along the ‘lower’ Cooper Creek (within South Australia) are outcrops of the Quaternary-aged Kutjitara and Katipiri Formations (Tedford and Wells 1990). Both formations were formed in a fluvial environment (Tedford and Wells 1990). The Kutjitara Formation is possibly middle Pleistocene in age based on thermoluminescence and electron spin resonance dating (see Alley 1998 and sources therein). Deposition of the Katipiri Formation mainly occurred in two periods around ~ 270–220 ka and ~ 120–90 ka (Callen and Nanson 1992; Nanson et al. 1992; Maroulis et al. 2007), with the youngest fossil-bearing deposits possibly being ~ 75–65 ka (see Magee et al. 1995).
Fossil accipitrids from the lower Cooper include a large distal tibiotarsus from Waralamanko Waterhole, specimens of Elanus scriptus (see Gaff 2002), and the type specimen (a lower left humerus) of Aviceda gracilis (de Vis 1905) (now considered an unidentified species of living Accipiter, see van Tets and Rich 1990; Gaff 2002).
Mairs Cave accipitrid
The presence of a very large, undescribed accipitrid in the fossil deposits of Mairs Cave has been acknowledged in multiple publications (Rich and van Tets 1982; Baird 1991; Baird et al. 1991). It was known from a nearly complete sternum, a distal humerus and two ungual phalanges that were collected in 1956 and 1969. This material was recovered roughly 55 m [60 yards] from the cave entrance, as per the collection data of ungual phalanx SAMA P.17139. Most specimens were covered in a thin layer of calcite, indicating that they had lain on the surface of the cave floor rather than being buried. The sternum and ungual phalanges were determined to represent an Old World vulture by Gaff (2002); at the time Aegypiinae and Gypaetinae were not separated into two clades. This material was also assessed by Mather (2021), who included the distal humerus and agreed with Gaff’s conclusion, with the additional clarification that the species was a gypaetine vulture.
On the 4th of December 2021, a team of Flinders University recreational speleologists and palaeontologists including EKM, ABC and THW revisited Mairs Cave to search for more fossil material of this accipitrid. Using the recorded location data associated with SAMA P.17139, the site of the original find was located and a further 28 bones were collected. These included the neurocranium, fragments of the mandible, several vertebrae, the extremitas sternalis and extremitas omalis of both sides of the furcula, proximal humerus, distal ulna, carpometacarpus, tibiotarsus shaft, tarsometatarsus, and several pedal phalanges from digits one, two and four. The bones were located scattered amid rocks against the south-eastern cave wall; a small concentration of bones represented the decomposition site, but several specimens had fallen about 3 m down through the rocks to a cave floor where periodic water flow had redistributed some up to several metres along the floor and enabled calcite deposition on them. Incredibly, a fragment of a distal humerus was found that was able to be reattached to the distal humerus that was collected over 50 years prior in 1969. Most of the material was very well-preserved, with many bones almost completely intact. With this discovery, the Mairs Cave accipitrid is now the best represented Australian fossil accipitrid from the Pleistocene. Additional fossils found in the vicinity of the accipitrid skeleton (and likely contemporaneous with it) have also resulted in expansion of the fossil fauna known from Mairs Cave (SI.1).
The aim of this study was to describe the new accipitrid species represented by the Mairs Cave skeleton and to assess its relationships. Additionally, isolated fossils from other sites are allocated to the same taxon, thereby extending its geographic and temporal range.
Materials and methods
Abbreviations
Institutes: Australian Museum, Sydney, New South Wales, Australia (AMF); Australian National Wildlife Collection, Canberra, Australian Capital Territory, Australia (ANWC); Flinders University, Adelaide, South Australia, Australia (FU/FUR); Museums Victoria, Melbourne, Victoria, Australia (NMV); South Australia Museum, Adelaide, South Australia, Australia (SAMA); Western Australian Museum, Perth, Western Australia, Australia (WAM); Smithsonian Museum of Natural History, Washington D. C., United States of America (USNM); University of Kansas Institute of Biodiversity (KU), Lawrence, Kansas, United States of America; Natural History Museum, London, UK (NHMUK); Canterbury Museum, Christchurch, New Zealand (CM).
Terminology
The terminology for the osteological features is derived from Baumel and Witmer (1993). Taxonomic nomenclature follows Dickinson and Remsen (2013) and Gill et al. (2020) for composition of Accipitriformes, and Nagy and Tökölyi (2014) for subfamilial composition.
Comparative material
Skeletons of living taxa were obtained on loan from museums and other institutions from across Australia and overseas as follows:
Outgroup. Threskiornithidae. Threskiornis spinicollis SAMA B48351. Ciconiidae. Ciconia ciconia SAMA B49223, SAMA B11601.
Accipitriformes. Cathartidae. Coragyps atratus SAMA B36873. Sagittariidae. Sagittarius serpentarius USNM 223836. Pandionidae. Pandion haliaetus SAMA B37096, NMV B30256. Accipitridae. Elaninae: Elanus axillaris NMV B34037; Elanus scriptus NMV B8617, NMV B30263, ANWC 22680; Gampsonyx swainsonii USNM 623110; Chelictinia ricourii NHMUK S.1904.4.28.3. Perninae: Elanoides forficatus USNM 622340; Chondrohierax uncinatus USNM 289784; Aviceda subcristata ANWC 22665, NMV B19826; Pernis apivorus SAMA B59278; Lophoictinia isura NMV B18533, ANWC 44373; Hamirostra melanosternon ANWC FALS-41, SAMA B36200. Gypaetinae: Polyboroides typus USNM 430434; Neophron percnopterus SAMA B11449; Gypohierax angolensis USNM 291316; Gypaetus barbatus NHMUK S.1972.1.59, NHMUK S.1896.2.16.120, NHMUK S.1952.3.61. Circaetinae: Spilornis cheela USNM 562001; Terathopius ecaudatus NMV B18575; Pithecophaga jefferyi NHMUK S.1910.2.11.1a, NHMUK S.1961.23.1. Aegypiinae: Necrosyrtes monachus USNM 620646; Gyps coprotheres ANWC 22724; Gyps fulvus NMV B18574, NMV B30269; Aegypius monachus NMV R553; Sarcogyps calvus NHMUK S.2013.22.1, NHMUK S.2007.30.1; Trigonoceps occipitalis NHMUK S.1954.30.54; Torgos tracheliotos NHMUK S.1930.3.24.248, NHMUK S.1952.1.172. Harpiinae: Harpia harpyja NHMUK S.1862.3.19, NHMUK S.1909.8.18.1. Aquilinae: Stephanoaetus coronatus NHMUK S.1954.30.42, NHMUK S.1862.3.14.19; Aquila audax SAMA B46613, NMV B19228; Aquila chrysaetos NMV B32659, ANWC 22682 (FALS-123); Aquila fasciata (labelled as Hieraaetus fasciatus) NMV B30575; Hieraaetus morphnoides SAMA B47128, NMV B8643, NMV B20224; Spizaetus tyrannus KU 35007; Spizaetus ornatus KU 72077. Haliaeetinae: Haliaeetus leucogaster NMV B8847, SAMA B49459; Haliaeetus leucocephalus ANWC 22723 (16500), NMV B15601; Haliaeetus albicilla NMV B34417; Haliastur indus ANWC 22719, NMV B13753; Haliastur sphenurus NMV B11661, SAMA B33998; Milvus migrans SAMA B47130, NMV B20404. Accipitrinae: Melierax metabates NHMUK S.1954.30.29; Kaupifalco monogrammicus NHMUK S.1869.10.19.28; Circus assimilis SAMA B56454, ANWC 22727; Circus approximans ANWC 22728, ANWC 22729; Circus cyaneus ANWC 22735; Circus aeruginosus NMV B12891; Accipiter fasciatus NMV B13444, SAMA B36355; Accipiter cooperii ANWC 22764, ANWC 22765; Accipiter striatus ANWC 22747, NMV B12666; Accipiter novaehollandiae NMV B18401; Accipiter cirrocephalus NMV B16071, NMV B10346; Accipiter nisus NMV B12413, ANWC 22742; Accipiter gentilis ANWC 22736, NMV B12927. Buteoninae: Erythrotriorchis radiatus NHMUK S.1872.10.22.9; Ictinia mississippiensis ANWC 22681 (21655), NMV B13343; Geranospiza caerulescens NHMUK S.1903.12.20.318; Buteo buteo SAMA B46558, NMV B24505; Buteo lagopus NMV B24884, ANWC 22776 (21,694); Buteo nitidus NMV B13222; Buteo rufofuscus NMV B24503.
In addition, casts of bones of the type series of the extinct Hieraaetus moorei (Haast, 1872) were examined as follows: NMV P33032 (tibiotarsus CM AV 5104pt); NMV P33031 (ungual phalanx CM AV 5104pt); NMV P33030 (tarsometatarsus CM AV 5104pt); NMV P33029 (femur, CM AV 5104pt); NMV P33028 (humerus, CM AV 5104pt); NMV P33027 (femur CM AV 5102pt); NMV P33026 (ulna CM AV 5104pt); and additional observations were made from figures in Holdaway (1991).
Measurements
Measurements were conducted using digital callipers, with results rounded to the nearest 0.1 mm.
Phylogenetic methods
A total of 300 morphological characters were compared in the fossil and selected living species, sampling from the following elements: neurocranium, sternum, scapula, humerus, ulna, carpometacarpus, digital phalanges, pelvis, femur, tibiotarsus, tarsometatarsus and pedal phalanges. Over half of these characters (154) were sourced from Migotto (2013), 2 from Elzanowski and Stidham (2010), 1 from Elzanowski and Zelenkov (2015), 8 from Gaff and Boles (2010), 1 from Worthy et al. (2016) and 3 from Mayr (2014), with the remaining 127 characters from Mather et al. (2021). The list of characters and character states can be viewed in SI.2. For the coding of the data, inapplicable characters were identified using ‘-’, though these are treated identically to missing data coded as ‘?’. Both the holotype and all referred fossil specimens were scored, with data available for viewing in SI.3.
Molecular data from Burleigh et al. (2015) were sourced for the following genes: cytochrome b, cytochrome oxidase 1, fibrinogen B beta introns 6–7, NADH dehydrogenase 2, RAG 1 and 12 s RNA. This was used to ensure that the relationships between the living species in this study would be resolved as accurately as possible. Sequences for the following species were sampled from the dataset of Burleigh et al. (2015): Ciconia ciconia, Coragyps atratus, Sagittarius serpentarius, Pandion haliaetus, Gampsonyx swainsonii, Elanoides forficatus, Chondrohierax uncinatus, Aviceda subcristata, Pernis apivorus, Lophoictinia isura, Hamirostra melanosternon, Polyboroides typus, Gypohierax angolensis, Neophron percnopterus, Gypaetus barbatus, Spilornis cheela, Terathopius ecaudatus, Necrosyrtes monachus, Torgos tracheliotos, Trigonoceps occipitalis, Gyps coprotheres, Gyps fulvus, Aegypius monachus, Sarcogyps calvus, Aquila chrysaetos, Hieraaetus morphnoides, Aquila fasciata, Hieraaetus moorei, Spizaetus tyrannus, Spizaetus ornatus, Haliaeetus leucogaster, Haliaeetus leucocephalus, Haliaeetus albicilla, Milvus migrans, Circus aeruginosus, Circus cyaneus, Accipiter cooperii, Accipiter striatus, Accipiter novaehollandiae, Accipiter gentilis, Buteo buteo, Buteo lagopus, Buteo rufofuscus, and Platalea leucorodia (as per Mather et al. 2021). Due to a lack of genomic data for Threskiornis spinicollis and Elanus caeruleus, data from Platalea leucorodia and Elanus scriptus were used, respectively, as stand-ins, as they are closely related taxa (see Campbell and Lapointe 2009 regarding usage).
Phylogenetic comparisons were aimed primarily at determining the relationships of the fossil species with respect to living taxa. A total of 47 species of modern Accipitridae, 1 species each of Pandionidae, Sagittariidae, Cathartidae, Threskiornithidae and Ciconiidae were sampled. The non-accipitrid species were selected for the following reasons: Pandionidae, Sagittariidae and Cathartidae are all members of the order Accipitriformes along with the Accipitridae; the Ciconiidae and Threskiornithidae are examples of bird families that fall outside of Accipitriformes but are similar in size and flight morphology, as well as having a history of grouping with the Cathartidae morphologically (Mather et al. 2021).
Parsimony and Bayesian phylogenetic analyses were used to analyse the data, and the main discussion focuses on combined morphology and molecular datasets, with morphology-only results in SI. The parsimony analyses of the morphological and combined morphological–molecular matrices were analysed using PAUP 4.0b10, using heuristic searches. Each search comprised 1000 random addition replicates, and enabled TBR branch swapping, with NCHUCK set to 1000 (see Mather et al 2021). The taxa Ciconia ciconia, Threskiornis spinicollis, Coragyps atratus and Sagittarius serpentarius were set as the most distal outgroup. Once the heuristic searches had generated a set of most parsimonious trees (MPT), a strict consensus tree was created from them. The clades formed by these trees were then assessed using bootstrapping (200 replicates), and the majority-rule consensus was set to a conlevel of 50 (only clades > 50% shown). Parsimony analyses were performed with Dynatoaetus alone added to the matrix with living taxa, and with both Dynatoaetus and Cryptogyps added.
For the Bayesian analyses, the software MrBayes 3.2.7 was used via the platform CIPRES (www.phylo.org). The analyses employed three partitions: the morphological data, and two DNA partitions, which were obtained from PartitionFinder (Lanfear et al. 2017) using the settings in Mather et al. (2021). These partitions were termed pfinder_molec1, which included Cyt-B codons 1 and 2, CO1 codons 1 and 2, ND2 codons 1 and 2, 12 s, Rag-1 codons 1, 2 and 3, and FGBint67; and pfinder_molec2, which included Cyt-B codon 3, CO1 codon 3, and ND2 codon 3. The Morph partition for discrete numerical states had among-character rate variability set to gamma, with distribution approximated using four categories. The Molec1 partition had the GTR model, with a Nst of 6, with substitution rates and frequencies having (separate) Dirichlet priors. The rates were set to InvGamma, with the gamma distribution approximated using four categories. The Molec2 partition also had a (separate) GTR model. The rates were set to Gamma, with the distribution approximated based on four categories. The number of MCMC chains was set to 4 (incrementally heated at 0.1), the number of generations set to 50,000,000, the sample frequency set to 5000. Each analysis was run four times (in parallel), and after suitable burnin was determined, a consensus tree was then derived from all four runs. Ciconia ciconia was initially set as the sole outgroup taxon, due to limitations with MrBayes, but trees were later re-rooted on Ciconia + Threskiornis. The Bayesian analyses included both Dynatoaetus and Cryptogyps.
The files containing the data matrix in PAUP and MrBayes format can be found in SI.4 and SI.5, respectively.
Results
Systematic palaeontology
Accipitriformes Vieillot 1816
Accipitridae Vigors 1824
Dynatoaetus gen. nov.
Zoobank ID : urn:lsid:zoobank.org:pub:236A128E-4FFD-4B0D-BEC1-3F6DF133E390
Diagnosis: A large accipitrid distinguishable from other genera by the following combination of characters: a neurocranium that is (1) relatively short and wide compared to Aquila audax; (2) has the line linking the tip of the processus zygomaticus to the ventral tip of the processus paroccipitalis aligned about 45 degrees to the plane of basioccipital-parasphenoidal plane and ventrally encloses a tympanic recess that is longer than high; (3) the condylus occipitalis is relatively large; (4) the mamillar tuberosities (tubercula basilaria) are robust, and prominent caudally; (5) the foramen magnum is near-perpendicular to the basioccipital plane (~ 10 degrees off perpendicular); (6) has prominent tubercula for the insertion for m. pseudotemporalis superficialis on the facies orbitalis. A sternum with (7) distinct and prominently projecting processus labrum internum; (8) a spina externa that is narrower than the apex carinae; (9) a crista medialis carinae that does not extend to the base of the spina externa. A humerus with (10) a weakly ventrally projecting epicondylus ventralis; (11) the distance between the interior margin of the tuberculum supracondylare ventrale and the proximal tip of the condylus dorsalis is equal to that between the tip of the condylus dorsalis and the dorsal margin; and (12) a deep insertion pit for the distal head of the m. pronator profundus. An ulna with (13) a tuberculum carpale that has little prominence cranioventrally. A tarsometatarsus that (14) is robust; (15) has a trochlea metatarsi II that is broad and more distally elongate than trochlea metatarsi III; (16) has the trochleae metatarsorum II and IV with robust plantar flanges and with (17) the plantar openings of the foramen vasculare proximale medialis positioned on the lateral side of the crista medialis hypotarsi; and (18) has the medial side of the shaft deeply excavated caudally by the fossa parahypotarsalis medialis such that the medial margin is very thin.
Etymology: The genus name is a combination of the ancient Greek words δῠνᾰτός (dynatós), meaning strong, mighty, or powerful, and ᾱ̓ετός (āetós) meaning eagle.
Type species: Dynatoaetus gaffae gen. et sp. nov.
Dynatoaetus gaffae gen. et sp. nov.
Zoobank genus ID : urn:lsid:zoobank.org:act:83F0032D-776F-4903-AC60-247C464F811F
Zoobank species ID : urn:lsid:zoobank.org:act:CC30C4DD-BFB5-4FED-87D9-ACE9323896AE
Diagnosis: Same as genus.
Holotype: SAMA P.59525 neurocranium, fragments of the mandible, two cervical vertebrae, three thoracic vertebrae, anterior end of the synsacrum, two caudal vertebrae, left and right sides furculum, right scapula, proximal right humerus, distal left ulna, proximal right radius, left carpometacarpus, left proximal phalanx major digit [or phalanx proximalis digiti majoris], two ribs, a tibiotarsus shaft, right tarsometatarsus, proximal right fibula, right metatarsal and left pedal phalanx I of digit I, left pedal phalanges I and II of digit II, right pedal phalanx I of digit IV, and an ungual phalanx; collected by Mather, Camens, Worthy et al. 4 December 2021. Ungual phalanx SAMA P.19157 and sternum SAMA P.19158, collected by CEGSA [Cave Exploration Group (South Australia)] June 1956; distal left humerus SAMA P.14528, collected by H. Mincham, 1969; ungual phalanx SAMA P.17139, collected by B. Daily and H. Mincham 1969—catalogue records “surface, under large rocks 60 yds in’’. All these specimens are part of a single skeleton (Fig. 2).
Visual representation of the parts of the skeleton known from Dynatoaetus gaffae (coloured grey). Image based on Meyer (1897), plate 121
Type locality and stratigraphy: Mairs Cave, Buckalowie Gorge, Flinders Ranges, SA, Australia, 32° 10′ 30 S, 138° 52′ 23 E (see Fig. 1). The bones of the Mairs Cave individual were 55 m from the entrance against the east wall of the main passage and were scattered between rocks over 3 m vertically and along the passage floor below the rockpile. It, therefore, was essentially a surface deposit and bones on the floor were associated with extinct Pleistocene mammal species.
Age of holotype: Pleistocene (Upper or Chibanian, precise age unknown).
Referred material: Main Fossil Chamber, Victoria Fossil Cave, Naracoorte, SA, Australia, − 37.0429°, 140.8016°, Pleistocene (Chibanian)—Right femur SAMA P.41514, excavation details [in metric feet from origin; see Reed 2003] 64.5–67.0, R9–10, D/D − 0.5 to − 1.0; right distal tarsometatarsus preserving trochleae II and III SAMA P.28008, excavation details 60.5–62.5, R1–2, D/D − 0.5 to − 1.0.
‘Old Collection’, Wellington Caves, NSW, Australia, 32° 31’ S, 148° 51’ E, Pliocene–Pleistocene—right distal tibiotarsus AM F.106562, likely acquired by NSW Mining Department 1884–1917.
Cooper Creek, Waralamanko Waterhole (referred to as ‘Waralamanka Waterhole’ in Gaff [2002]), site A, Kutjitara Formation, Lower Cooper Fauna, Pleistocene—right distal tibiotarsus SAMA P.25218, specimen, currently unlocated, was depicted in Plates 5.1 and 5.2 Gaff (2002).
Etymology: The name ‘gaffae’ honours Priscilla Gaff, who first discussed the fossil material of this species in her 2002 thesis revising Australian accipitrids.
Measurements (mm): neurocranium (SAMA P.59525): length from nasofrontal hinge (marked by preserved sulcus for processus frontalis of premaxilla) to condylus occipitalis 67.3, preserved height from the medial processes of the lamina parasphenoidalis to the dorsal margin of the skull 49.1; interorbital width 32.3, estimated width at postorbital processes 60–62, estimated width across squamosals 62.0, width between fossae temporales 51.7, width across processus paroccipitales 49.6; mandible (SAMA P.59525): maximum width cotyla lateralis to tip processus medialis 28.0, width across cotyla lateralis et medialis 19.5; sternum (SAMA P.19158): preserved cranial width (between lateral margins of processus craniolaterales) 69.0, height apex carinae (from apex to cranial margin of spina externa) 70.8, pila carinae minimum width 7.8, depth sternum [maximal basin depth between costal margins] 51.0; scapula (SAMA P.59525): cranial width 27.5, width between facies articularis humeralis and the acromion 30.5, length facies articularis humeralis 16.1, depth collum scapula 13.1; distal humerus (SAMA P.14528): preserved least shaft width 24.8, preserved distal width 45.1 (from epicondylus medialis to epicondylus dorsalis); proximal humerus (SAMA P.59525): proximal width tuberculum dorsale to crista bicipitalis 53; ulna (SAMA P.59525): maximum distal width 24.4, width in cranial aspect between tuberculum carpale and ventral face of condylus dorsalis ulnaris 18.2, preserved maximum shaft width 13.6 (dorsal aspect), minimum shaft width 12.5 (cranial aspect); carpometacarpus (SAMA P.59525): preserved total length 130.4 (~ 3 mm of articular process of phalanx digiti minoris missing), length to facies articularis major 128.0, maximum proximal width 35.4, midshaft width 8.6, preserved distal width 23.8; femur (SAMA P.41514): length 145.5, maximum proximal width 38.3, preserved proximal depth trochanter 31.4, shaft width 20.5 (cranial view), least shaft circumference 69, maximum distal width 39.8 (including epicondylus lateralis), width condylus medialis 18.7, proximodistal length condylus medialis 22.5, depth condylus medialis 29.3, width condylus lateralis 17.8, proximodistal length condylus lateralis 22.4, depth condylus lateralis 34.8; tibiotarsus (AM F.106562): preserved length 97.8, least shaft circumference 53.0, least shaft width 14.6, maximum distal width 27.8, craniocaudal depth of condyles 16.3 (preserved); distal tibiotarsus (SAMA P.25218): Gaff (2002) recorded distal width as 27.1 mm; tarsometatarsus (SAMA P.59525): total length (axial length eminentia intercotylaris to distal margin trochlea metatarsi II) 136.7, proximal width 30.4, preserved proximal depth 22.0 (crista medialis hypotarsi is missing about 1/4 of depth), proximal depth at sulcus hypotarsi 13.2, midshaft width 15.3, maximum distal width 34.5 (includes plantar flanges on trochleae), trochlea metatarsi II plantar width 14.0 (includes flange), trochlea metatarsi II depth 16.1 (flange is 4.1 of depth), trochlea metatarsi III width 10.5, trochlea metatarsi III depth lateral side 16.1, trochlea metatarsi IV width 7.2, trochlea metatarsi IV depth 18.6 (flange is 5.1 of depth); tarsometatarsus (SAMA P.28008): width trochlea metatarsi II 8.8, depth trochlea metatarsi II 12.4, width trochlea metatarsi III 9.9, depth trochlea metatarsi III 14.8; Os metatarsale I (SAMA P.59525): preserved length 30.0, distal width 18.4; pedal phalanx I.1 (SAMA P.59525): preserved length 33.8, proximal width 24.7, shaft width 11.8; pedal phalanx II.1 (SAMA P.59525): length 16.3, width 21.1; pedal phalanx II.2 (SAMA P.59525): length 35.3, proximal width 15.3, shaft width 11.3, distal width 11.9; pedal phalanx IV.1 (SAMA P.59525): length 19.5, proximal width 10.5, proximal height 11.2, shaft width 9.2, distal width 7.7; ungual phalanx (SAMA P.59525): length 35.8, articular facet height 16.0, articular facet width 13.4, tuberculum flexorium height 9.2, tuberculum flexorium width 8.7, tuberculum flexorium length 8.7; ungual phalanx (SAMA P.17139): length 37.1, articular facet height 15.4, width articular facet 12.9, height tuberculum flexorium 10.3, width tuberculum flexorium 7.9, length tuberculum flexorium 8.8; ungual phalanx (SAMA P.19157): length 35.5, height articular facet 16.1, width articular facet 12.5, height tuberculum flexorium 9.4, width tuberculum flexorium 9.5, tuberculum flexorium length 8.7. Comparative measurements with specimens of Aquila audax and Gyps coprotheres can be viewed in SI.6. A visual comparison of the tarsometatarsus and distal humerus to the same elements in Aquila audax, Torgos tracheliotos, Sarcogyps calvus, Trigonoceps occipitalis and Gypaetus barbatus can be found in SI.7.
Comparisons with Australasian Pleistocene taxa
Dynatoaetus gaffae can be separated from the aegypiine Cryptogyps lacertosus by the following features: it is significantly larger than C. lacertosus; the distance between the proximal tip of the condylus dorsalis and the margin of the tuberculum supracondylare ventrale on the humerus is equal to the distance between the condylus dorsalis and the lateral margin of the face between the tuberculum supracondylare dorsale and the epicondylus dorsalis (greater in C. lacertosus); the tarsometatarsus is moderately elongate (stout in C. lacertosus), the sulcus hypotarsi is broad (narrow), the base of the cristae hypotarsi are connected to a flat ventral surface (connected to a raised ridge), the foramen vasculare proximale medialis is located lateral to the crista medialis hypotarsi (located medial to the crista), the impressiones retinaculi extensorii form two prominent ridges with a deepened depression between them (ridges absent, no deepening), and the medial shaft is very thin dorsoplantarly (thick).
Dynatoaetus gaffae is only matched in size in the Australasian region by the extinct New Zealand eagle Hieraaetus moorei, but can be distinguished from the latter via the following features (Hieraaetus state in brackets): the sternum has prominent processus labrum internum present (absent/very short in H. moorei); in the humerus, the incisura capitis is shallow (deep in H. moorei), the palmar insertion for the m. extensor metacarpi radialis forms a distinct line (circle-shaped in H. moorei), the ventral margin of the fossa forms a curved line parallel to the shaft margin (straight in H. moorei), the distance between the ventral margin of the fossa brachialis and the ventral shaft margin is between 1/3 and 1/4 shaft width (less than 1/4 shaft width in H. moorei), the distance between the tip of the condylus dorsalis and the margin of the tuberculum supracondylare ventrale is equal to the distance between the condylus dorsalis and the lateral margin of the face between the tuberculum supracondylare dorsale and the epicondylus dorsalis (greater width in H. moorei), the interior margin of the tuberculum supracondylare ventrale is oriented at least 45–50° across the shaft (oriented parallel to the shaft in H. moorei); in the ulna, the tuberculum carpale is weakly projecting cranially (moderate projection into a distinct peak); in the tarsometatarsus, the foramen vasculare proximale medialis is located lateral to the crista medialis hypotarsi (located medial to the crista) and is positioned adjacent to the base of the crista (positioned proximal to the base of the crista), the tuberositas m. tibialis cranialis is directly distal to the foramina vascularia proximalia on the dorsal facies (well separated distally), the impressiones retinaculi extensorii form two prominent ridges with a deepened depression between them (ridges absent, no deepening), the impressio lig. collateralis lateralis forms a prominent inflation on the lateral facies (flattened scar), and the second and third trochleae have equal distal extent (third trochlea longer distally). Thus, Dynatoaetus gaffae differs substantially from H. moorei.
Comparative descriptions of Dynatoaetus gaffae with living taxa
Neurocranium (Fig. 3A–E)
Dynatoaetus gaffae SAMA P.59525 neurocranium in dorsal (A), ventral (B), lateral (C), left antero-lateral (D), and caudal (E) view, and mandible fragments (F). CO condylus occipitalis, COD crista otica dorsalis, FM foramen magnum, LP lamina parasphenoidalis, LPMP tuberculum basilare, PM processus medialis, PrPa processus paroccipitalis, PrPO processus postorbitalis, PrZ processus zygomaticus, SI interorbital sulcus, SO sulcus n. olfactorius. Lines on D frame tympanic cavity relative to basioccipital plane. Scale bar is 50 mm
The neurocranium is covered in a thin layer of calcite. It is preserved anteriorly from the craniofacial hinge as revealed by the preserved sulcus for the right side of processus frontalis of the premaxilla. Anteriorly, breakage results in the lacrimal articular facets not being preserved; similar both processus postorbitales (Fig. 3, PrPO) are broken. Dorsocaudally, a combination of corrosion and abrasion has removed the prominentia cerebellaris and areas lateral of it, although the foramen magnum is entire. The right squamosal area is damaged such that squamosal width is compromised. The following features are observable:
There is an interorbital sulcus (Fig. 3, SI) extending caudally from the craniofacial hinge to the mid-length of the orbits. The lacrimal articular zone, while the facet is not preserved, must be relatively short caudal to the sulcus for the processus frontalis, and not more than 12 mm of the orbit length of ~ 35 mm, suggesting that it was relatively small. Relative to the plane of the basioccipital-parasphenoid, the cranial cavity is tilted about 40 degrees (Aquila is about 60 degrees) and related to this is the steep plane of the foramen magnum (Fig. 3, FM) (~ 80 degrees to basioccipital plane) and the shallow angle of the line joining in the processus zygomaticus (Fig. 3, PrZ) and the ventral tip of the processus paroccipitalis (Fig. 3, PrPa). This results in the cavum tympanicum being rather longer than it is high. A raised facet within the tympanic cavity that buttresses the caudal side of the otic capitulum of the quadrate (when it is articulated) appears relatively low and small compared to that of A. audax, and similar to the condition observed in Aegypius and Gyps. However, this may be exaggerated by breakage.
The prominentia cerebellaris was probably weakly differentiated from the rest of the skull (Gypaetinae, most Aegypiinae, Circaetinae, Harpiinae), though the degree of this may be exaggerated by damage to this region. The foramen magnum is positioned caudoventrally on the caudal end of the skull (some species of Gypaetinae, Aegypiinae) rather than caudally as in most Accipitridae. The condylus occipitalis (Fig. 3, CO) is very large relative to the size of the skull implying robust proximal cervical vertebrae. The lamina parasphenoidalis (Fig. 3, LP) is unfused (some Perninae, Gypaetinae, some Circaetinae, Aquilinae, some Haliaeetinae, some Buteoninae). The tuberculum basilare (Fig. 3, LPMP) is short but prominently protruding ventrally in caudal view (some Perninae, Gypaetinae, most Aegypiinae, Circaetinae, Harpiinae, Haliaeetinae). The crista otica dorsalis (Fig. 3, COD) is laterally expanded (Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Harpiinae, Haliaeetinae, some Buteoninae (Buteo)). Prominent tubercula are present on the caudal section of the area muscularis aspera, medial to the processus postorbitalis (Gypaetinae). The processus paroccipitalis distinctly projects caudally (Gypaetinae, Buteoninae), though not to as great a degree as seen in the Aegypiinae. The neurocranium height is estimated to be roughly 80% of its width (most similar to Gypaetinae, Aegypiinae).
Mandible (Fig. 3F)
The rostral tip and the left articular end of the mandible are preserved. The following features can be observed:
The processus medialis (Fig. 3, PM) is short but prominently protruding medially. The articular area across the lateral and medial cotylae is robust, ~ 19.5 mm wide, forming an articular surface wider than long (most Accipitridae except Aegypiinae).
Vertebrae (Fig. 4)
Seven vertebrae are preserved, which include three thoracic vertebrae (Fig. 4A–C), two caudal vertebrae (Fig. 4D, G), and two cervical vertebrae (Fig. 4F, G). Due to their limited number, their precise position along the spine is difficult to determine.
Sternum (Fig. 5A–C)
Dynatoaetus gaffae sternum (SAMA P.19158) in cranial (A), dorsal (B) and lateral view (C), scapula (SAMA P.59525) in medial (D) and lateral view (E), and furcula (SAMA P.59525) fragments (F). AC apex carinae, Acr acromion, AF apophysis furculae, CMC crista medialis carinae, CS corpus scapulae, EOC extremitas omalis claviculae, F foramen, FAA facies articularis acrocoracoidea, FAC facies articularis clavicularis, FAH facies articularis humeralis, FS facies muscularis sterni, PA processus acromialis, PC processus costalis, PL processus labra internum, SAC sulcus articularis coracoideus, SE spina externa, T tuberculum, TC tuberculum coracoideum. Scale bar is 50 mm
The cranial half of the sternum is well-preserved including the pila carinae, labrum internum, both processus labrum internum, sulcus coracoidei, processus costales, and pars cardiaca, while the spina externa is present but damaged. The following features can be observed:
The spina externa (Fig. 5, SE) is short (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae), and appears to have been narrower than the apex carinae. The left sulcus articularis coracoideus (Fig. 5, SAC) overlaps the right sulcus articularis coracoideus immediately dorsal to the spina externa (Perninae, Gypaetinae [excluding Gypaetus], Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). A pair of processus labra internum (Fig. 5, PL) are present (Perninae, most Gypaetinae, few Aquilinae, Haliaeetinae, Buteoninae), and are robust and cranially prominent (some Perninae, some Gypaetinae). The crista medialis carinae (Fig. 5, CMC) does not extend to the base of the spina externa (only observed in Aegypiinae). In lateral view, the apex carinae (Fig. 5, AC) is positioned caudal to the cranial margin of the sulcus articularis coracoideus (Perninae, Gypaetinae, Aegypiinae). The apex carinae is of roughly the same width as the pila carinae (Perninae, Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). Five processus costales are preserved and a sixth likely was present more proximally (Perninae [excluding Elanoides], Gypaetinae, Aegypiinae). The pars cardiaca has very little pneumatisation, with no distinct foramen pneumaticum (Perninae, Gypaetinae, Circaetinae, Aegypiinae, Haliaeetinae). There is no foramen present in the facies muscularis sterni (Fig. 5, FS) (Perninae, Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The margin of the facies muscularis sterni extends to the (assumed) fourth processus costalis (Gypaetinae, some species of Aegypiinae, some species of Circaetinae, Haliaeetinae). The carina depth (measured from the dorsal tip of processus costales to the ventral base of pars cardiaca) is less than that of the sternal basin (Perninae, Gypaetinae, Aegypiinae, some Circaetinae).
Scapula (Fig. 5D, E)
The cranial two-thirds of the right scapula is preserved with little damage. The following features can be observed:
With the collum horizontal, the acromion (Fig. 5, Acr) projects prominently cranially relative to the tuberculum coracoideum (Fig. 5, TC) and the facies articularis humeralis (Fig. 5, FAH) (majority of Accipitridae) and in cranial aspect widens dorsally, with the facies articularis clavicularis (Fig. 5, FAC) more dorsally prominent than the medial side and crista ligamentum acrocoracoacromiali. There is no foramen or pneumatisation present in the cranial region of the acromion (as in most Perninae, Gypaetinae [Gypaetus barbatus], some Circaetinae, some Haliaeetinae). The tuberculum coracoideum is low and discrete, barely projecting cranially (Perninae, Gypaetinae, Aegypiinae, Haliaeetinae, Buteoninae). A small foramen (Fig. 5, F) is present in the medial facies of the cranial end (some Perninae, some Circaetinae, some Aquilinae, some Haliaeetinae). The ventral margin of the facies articularis humeralis smoothly joins with the shaft margin, not being ventrally offset, or notched (Perninae). The dorsal margin of the corpus scapulae (Fig. 5, CS) starts to expand dorsally 47 mm caudal of the acromial tip, so that depth is greater more caudally than at the collum (Perninae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae).
Furcula (Fig. 5F)
The left and right scapus claviculae and the fused extremitas sternalis claviculae of the furcula are preserved in three pieces. The following features can be observed:
The scapus claviculae are broad (29 mm wide) with robust facies articularis acrocoracoidea (Fig. 5, FAA). The extremitas omalis claviculae (Fig. 5, EOC) have large, circular impressions which are pneumatic (some Aegypiinae, most Gypaetinae, Haliaeetinae). The distance between the processus acromialis and the ventral facies of the articularis acrocoracoidea is relatively short (31 mm), which equals width at the base of the processus (Gypaetinae, Aegypiinae, Haliaeetinae). The apophysis furculae (Fig. 5, AF) is worn but its gracile nature suggests it had very little ventral projection (Perninae, Gypaetinae, Haliaeetinae). The furcula forms a broad U-shape in dorsal aspect (Perninae, Gypaetinae, Haliaeetinae). There is little to no deepening of the cranial facies directly dorsal to the apophysis furculae (Gypaetinae, Aegypiinae).
Humerus (Fig. 6A–D)
Dynatoaetus gaffae (SAMA P.59525) right proximal humerus in caudal (A) and cranial (B) view, plus left distal humerus (SAMA P.14528) in cranial (C) and ventral (D) view. CDm condylus dorsalis margin, ED epicondylus dorsalis’, EV epicondylus ventralis, FB fossa brachialis, FP fossa pneumotricipitalis, IC incisura capitis, MEMR scar for m. extensor metacarpi radialis, MEMU scar for m. extensor metacarpi ulnaris, MPP scar for m. pronator profundus, MPS scar for m. pronator superficialis, MSC scar for m. scapulohumeralis caudalis, SLT sulcus lig. transversus, TD tuberculum dorsale, TSD tuberculum supracondylare dorsale, TSV tuberculum supracondylare ventrale. Scale bar is 50 mm
A proximal right humerus preserves the tuberculum dorsale on the dorsal rim, the caput, most of the intumescentia humeri, the tuberculum ventrale (except its tip), the fossa pneumotricipitalis ventralis and the crus ventrale fossa, but the crista bicipitalis, the crista deltopectoralis and all parts of the shaft are not preserved. The distal shaft and proximal-most section of the left distal end are preserved, including the fossa brachialis, insertion scars for the m. extensor carpi radialis, tuberculum supracondylare dorsale, the epicondylus ventralis and tuberculum supracondylare ventrale. The distal condyles are completely broken off. Among several fragments recovered in 2021 was the epicondylus ventralis, which articulated cleanly with the larger fragment collected in 1969 and was reaffixed. The following features could be observed:
The incisura capitis (Fig. 6, IC) is shallow (Gypaetinae, Aegypiinae) and the caput has low convexity in cranial view (some Perninae, some Aegypiinae, Buteoninae). The ventral section of the sulcus lig. transversus (Fig. 6, SLT) forms a deep impression but is constricted where it opens to the ventral facies (Perninae, Gypaetinae, Circaetinae, Haliaeetinae). The tuberculum dorsale (Fig. 6, TD) is positioned level with this sulcus, with very little dorsal projection evident (some Perninae [Elanoides forficatus, Hamirostra melanosternon], most Gypaetinae, some Circaetinae [Terathopius ecaudatus], Aegypiinae, Haliaeetinae, some Buteoninae). The fossa pneumotricipitalis (Fig. 6, FP) is occluded by sediment but is narrow, 13 mm (25%) of proximal width, and bound ventrally by a robust crus ventrale fossa (Perninae, some Gypaetinae, some Circaetinae, Aquilinae, most Haliaeetinae (excluding Haliaeetus), Buteoninae). The insertion scar for the m. scapulohumeralis caudalis (Fig. 6, MSC) is level with (neither sunken nor elevated) the surface of the crus ventral fossa (Perninae, Gypaetinae, some Aegypiinae, Aquilinae (excluding Aquila), Haliaeetus (Haliaeetinae)).
Distally, the fossa brachialis (Fig. 6, FB) has a deepened, circular ventral scar, with a shallow section extending proximodorsally from it (Perninae, Gypaetinae, Aegypiinae, Haliaeetinae). The fossa brachialis is not pneumatic (all Accipitridae). The dorsal margin of the fossa brachialis is broadly separated from the shaft margin (9 mm) by a distance less than a quarter of the fossa width (some Perninae, some Gypaetinae, Aegypiinae). The ventral margin of the fossa is curved and is not parallel to the shaft margin (Gypaetinae, Circaetinae). The palmar insertion scar for the m. extensor metacarpi radialis (Fig. 6, MEMR) is a broad (9 mm wide dorsoventrally), oval shape and separated from the condylus dorsalis by ~ 6 mm. The dorsal insertion scar for the m. extensor metacarpi radialis is small, shallow and circular/round (Perninae, Gypaetinae, Circaetinae, Buteoninae) and is only slightly prominent dorsally of the shaft proximal to it. Thus, the tuberculum supracondylare dorsale (Fig. 6, TSD) is low (few Perninae, some Gypaetinae, some Circaetinae, some Aegypiinae, Harpiinae, few Aquilinae). The dorsal profile expands dorsally more distally, such that the epicondylus dorsalis (Fig. 6, ED), even in its incompletely preserved state, was more prominent dorsally than the tuberculum supracondylare dorsale (some Gypaetinae, Aegypiinae). The interior margin of the tuberculum supracondylare ventrale (Fig. 6, TSV) is aligned at a 45–50° proximodorsally across the shaft, and the proximal margin of the tuberculum prominently projects cranially (most Perninae, Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The distance between the interior margin of the tuberculum supracondylare ventrale and the proximal tip of the condylus dorsalis (Fig. 6, CD) is roughly equal to the distance between the tip of the condylus dorsalis and the dorsal margin (some Perninae, Gypaetinae). The proximal insertion for the m. pronator superficialis (Fig. 6, MPS) is large, circular, deep, and close to the tuberculum supracondylare ventral (some Perninae, Circaetinae, Aquilinae, Haliaeetinae). The distal insertion for the m. pronator superficialis is deep, distinct, and located adjacent to the ventrodistal end of the tuberculum. The epicondylus ventralis (Fig. 6, EV) has very little ventral prominence relative to the shaft, such that the shaft profile is straight level with the tuberculum supracondylare ventrale, rather than concave (Perninae, some Gypaetinae). On the epicondyle, the insertion for the m. pronator profundus (Fig. 6, MPP) is deeper than the insertion for the m. extensor metacarpi ulnaris (Fig. 6, MEMU) (most Perninae, Gypaetinae, Circaetinae, Aegypiinae, Aquilinae).
Ulna (Fig. 7C, D)
Dynatoaetus gaffae (SAMA P.59525) left carpometacarpus in dorsal (A) and ventral (B) aspect, distal left ulna in dorsal (C) and caudal (D) aspect, proximal right radius in dorsal (E) aspect, phalanx distalis digiti majoris I (F), and phalanx digiti alulae (G). CD condylus dorsalis, FADA facies articularis digiti alulae, OMMi os metacarpale minus, PE processus extensorius, PP processus pisiformis, SI sulcus intercondylaris, ST sulcus tendinosus, TC tuberculum carpale. Scale bars: A–C is 50 mm, E–G is 10 mm
The distal end of the left ulna is well-preserved but is covered in a thin layer of calcite. The following features are notable:
The tuberculum carpale (Fig. 7, TC) barely projects cranioventrally. The sulcus intercondylaris (Fig. 7, SI) is deep and V-shaped (most Accipitridae). The proximal margin of the condylus dorsalis (Fig. 7, CD) does not project ventrally and connects to the shaft in a continuous line in caudal view (some Gypaetinae, Aegypiinae). The length of the condylus dorsalis (24.7 mm, 1–2 mm missing) is slightly greater than its craniocaudal depth (22.5) in dorsal aspect (some Perninae, Aegypiinae, Circaetinae, Aquilinae).
Carpometacarpus (Fig. 7A, B)
The entire left carpometacarpus is preserved except for the tip of the articular process for phalanx digiti minoris, with the ventral surface coated with a thin veneer of calcite. The following features can be observed:
There is no pneumatisation in any region of the proximal end (all Accipitridae except Aegypiinae). The processus extensorius (Fig. 7, PE) projects cranially 3 mm of 35.5 total width (similar in Circaetinae, Aquilinae, Haliaeetinae, Buteoninae) with its proximal margin at right angles to the shaft of the trochlea carpalis (Fig. 7, TC) (most Perninae, Gypaetinae, Circaetinae, Aegypiinae, Harpiinae, Haliaeetinae). The facies articularis digiti alulae (Fig. 7, FADA) abuts the shaft (most Perninae, Gypaetinae). The distal-most point of the trochlea carpalis is in line with the base of the processus pisiformis (Fig. 7, PP) (majority of Accipitridae). The sulcus separating the processus pisiformis and processus extensorius is shallow (Perninae, some Gypaetinae (Gypaetus), some Aegypiinae, some Haliaeetinae, Buteoninae (Buteo)). The proximal region of the os metacarpale minus (Fig. 6, OMMi) is deeply concave (majority of Accipitridae). The distal end of the sulcus tendinosus (Fig. 7, ST) (for musculus extensor digitorum communis, Stegmann 1978) is bordered by a low ridge on the cranial side of the groove (similar to, but not quite as flattened as Aegypiinae). There is a distinct shallow tendinal sulcus diverging from the main tendinal sulcus onto the cranial facies that is interpreted as for musculus extensor indicus longus (Stegmann 1978) (Circaetinae, Aquilinae, Haliaeetinae, Buteoninae).
Phalanx proximalis digiti majoris (Fig. 7F)
The length between articular surfaces is 55.7 mm; the caudal margin is broken so its width is indeterminate.
Femur (Fig. 8A-D)
Right femur from Victoria Fossil Cave (SAMA P.41514) assigned to Dynatoaetus gaffae, in cranial (A), lateral (C) and caudal (D) aspect, with an illustration of the musculature scars on the lateral side of the proximal end (B). A right distal tibiotarsus (AM F.106562) from the Wellington Caves in cranial (E), lateral (F) and medial (G) aspect. CL condylus lateralis, CM condylus medialis, CSM crista supracondylaris medialis, CT crista trochanteris, CTib crista tibiofibularis, DLER distal/lateral insertion of extensor retinaculum, EL epicondylus lateralis, EM epicondylus medialis, F foramen, FLC fovea lig. capitis, FP fossa poplitea, IGI impressio gastrocnemialis intermedia, IGL impressio gastrocnemialis lateralis, II incisura intercondylaris, ILCL impressio lig. collateralis lateralis, LIC linea intermuscularis cranialis, MICa m. iliotrochantericus caudalis insertion scar, MICr m. iliotrochantericus cranialis insertion scar, MIE m. ischiofemoralis extensorius insertion scar, MIM m. iliotrochantericus medius insertion scar, MOL m. obturatorius lateralis insertion scar, MOM m. obturatorius medialis insertion scar, PCa impressio lig. cruciati caudalis, PCr impressio lig. cruciati cranialis, PMER proximal/medial insertion of extensor retinaculum, PS pons supratendineus, SE sulcus extensorius, SF sulcus m. fibularis, SFA spina fibulae, SI sulcus intercondylaris, SP sulcus patellaris. Scale bar is 50 mm
The femur SAM P.41514 is referred to this species based on its appropriate and distinctive large size and co-occurrence with SAMA P.28008, a partial tarsometatarsus identical to the holotype tarsometatarsus specimen.
The femur is extremely large and robust compared to all living Australasian accipitrids (see measurements), with only specimens of the extinct Haast’s eagle Hieraaetus moorei outsizing it. The fovea lig. capitis (Fig. 8, FLC) is deep (Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae) and large relative to the caput (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The fossa trochanteris is very shallow (Perninae, most Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The crista trochanteris (Fig. 8, CT) has a little breakage affecting the proximocranial part, but is low (most Perninae, Aquilinae, Haliaeetinae, Buteoninae), the cranial surface medial to it is flat rather than concave (Perninae, some Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae), and it has one relatively small pneumatic foramen (Fig. 8, F) penetrating it medially in its distal third of length (some Gypaetinae, some Aegypiinae, Circaetinae, Buteoninae). The depression distad to the facies articularis antitrochanterica on the caudal face is very shallow (Perninae, most Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae). The linea intermuscularis cranialis (Fig. 8, LIC) is positioned laterally (most Gypaetinae, Aegypiinae, Circaetinae). Laterally on the proximal end, the insertions of various muscles are well-marked. Using the terminology from Matsuoka and Seoka (2021), they are as follows: The scar for the insertion of m. obturatorius medialis (Fig. 8, MOM) occupies a 9 mm wide bulge level with the collum and in the caudal half of the lateral facies, with radial scarring present across a continuous area of 16.2 mm. The insertion of the m. obturatorius lateralis (Fig. 8, MOL) forms a deep oval scar distal to the m. obturatorius medialis (similar to, but much deeper and more distinct than, Aquilinae and Haliaeetinae). More distally a larger and deep scar centred on the shaft is for the insertion of the m. ischiofemoralis extensorius (Fig. 8, MIE) (most Accipitridae). The linear insertion scar of m. iliotrochantericus caudalis (Fig. 8, MICa) is about 18 mm long and runs parallel to and about 6 mm caudal to the crista trochanteris (similar to Perninae, Gypaetinae, Aegypiinae); it is well separated from the margin of the crista trochanteris (Perninae, Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). Distally, this scar ends adjacent to a small round scar for the insertion of m. iliotrochantericus medius (Fig. 8, MIM) (Perninae, Gypaetinae, Aegypiinae). The insertion scar of m. iliotrochantericus cranialis (Fig. 8, MICr) is the most distal and is centred on the shaft distally-adjacent to the scar for the insertion of m. ischiofemoralis (Necrosyrtes (Aegypiinae)).
These differ from sympatric Aquila audax in details as follows (Aquila in brackets): that for m. obturatorius medialis is elevated (in depression) and forms one large, continuous scar (split into several smaller attachment points); that for m. iliotrochantericus caudalis is longer ending distally level with m. iliotrochantericus caudalis (ends distinctly more proximally); m. iliotrochanterici medius is rounded (distinctly elongate and extends more proximally); m. ischiofemoralis is well separated from m. iliotrochanterici medius (close together).
The proximal point of the epicondylus lateralis (Fig. 8, EL) is strongly projecting laterally (most Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae). The crista supracondylaris medialis (Fig. 8, CSM) is prominent as formed by the tuberculum muscularis gastrocnemialis medialis. The impressio gastrocnemialis lateralis (Fig. 8, IGL) is shallow and large (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Haliaeetinae, Buteoninae) but is not visible in caudal aspect as it is entirely on the lateral facies and set cranial to the epicondylus lateralis. The condylus medialis (Fig. 8, CM) in caudal view is robust and distally wide and well-rounded (much narrower in Aquila), resulting in a relatively narrower notch distally for the sulcus intercondylaris (Fig. 8, SI). The condylus lateralis and medialis are separated by a deep notch caudally (Perninae, Aegypiinae, Aquilinae, Haliaeetinae, Buteoninae). The impressio gastrocnemialis intermedia (Fig. 8, IGI) is circular in shape (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Haliaeetinae, Buteoninae), and is positioned centrally in the shaft at the proximal edge of the fossa poplitea (Fig. 8, FP) (most Perninae, most Gypaetinae, Circaetinae, most Aquilinae). The fossa poplitea is deep (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Aquilinae). The impressio lig. cruciati caudalis et cranialis form two distinct shallow sulci (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Buteoninae). The impressio lig. cruciati cranialis (Fig. 8, PCr) is relatively twice as wide as the impressio lig. cruciati caudalis (Fig. 8, Pca) (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Buteoninae). The impressio lig. cruciati caudalis is slightly deeper than the impressio lig. cruciati cranialis (Perninae, Circaetinae, Aquilinae). The impressio lig. collateralis lateralis (Fig. 8, ILCL) spans roughly two-thirds the caudo-cranial depth of the condylus lateralis (Perninae, Aegypiinae, most Aquilinae, Buteoninae). The crista tibiofibularis (Fig. 8, Ctib) on the condylus lateralis is very robust (most Accipitridae). The sulcus patellaris is relatively short, with the length in cranial view being ~ 30 mm compared to a distal width of 40.4 (some Perninae, some Gypaetinae, Circaetinae, Aegypiinae).
Tibiotarsus (Fig. 8E–G)
The holotype specimen from Mairs Cave preserves only a shaft of a tibiotarsus. However, two distal tibiotarsi can be referred to the new species by appropriate large size and fit with the preserved proximal tarsometatarsus of the Mairs cave specimen: a distal tibiotarsus from the Wellington Caves (AM F.106562); a distal tibiotarsus from Waralamanka Waterhole, Cooper Creek (SAMA P.25218) (see Gaff 2002 Figs. 5.1, 5.2 that show its similar size and form to AM F.106562). They are much larger than would be predicted for Cryptogyps lacertosus based on the size of the tarsometatarsus referred to that taxon. They are also much larger than those of the living Aquila audax and Haliaeetus leucogaster. On AM F.106562, the distal end is well-preserved except for the condylar margins, which are pitted and worn, especially caudally, where the condyles are completely eroded. The AM F.106562 tibiotarsus reveals the following features:
The width (27.5 mm) of the distal end is much greater than its depth (18.1 mm, estimated original depth 19 mm) (similar to Perninae, Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The distal margin of the pons supratendineus (Fig. 8, PS) is angled between 30–40° relative to the alignment of the shaft (most Gypaetinae, Aegypiinae, Circaetinae, most Haliaeetinae). The cranial proximal margin of the condylus lateralis (Fig. 8, CL) is level with the distal-most margin of the pons supratendineus (Perninae, Gypaetinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The proximomedial side of the pons is widely separated from the medial margin of the shaft (most Perninae, Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The proximomedial scar for the extensor retinaculum (Fig. 8, PMER) is not raised and is positioned just proximal to the pons (Perninae, Aegypiinae, Circaetinae, most Aquilinae, Haliaeetinae (Haliaeetus)). The distal lateral scar for the extensor retinaculum (Fig. 8, DLER) is positioned at the disto-lateral end of the pons (Perninae, Gypaetinae, Aegypiinae, Circaetinae, Aquilinae, Haliaeetinae, Buteoninae), not more laterally. The sulcus m. fibularis (Fig. 8, SF) is on the lateral facies and deep (Gypaetinae, some Circaetinae, Aquilinae, Haliaeetinae). About half the condylus medialis (Fig. 8, CM) is displaced medially past the shaft margin (Aegypiinae), rather than entirely mesad. The incisura intercondylaris (Fig. 8, II) is shallow (Perninae, most Gypaetinae, Aegypiinae, Circaetinae, Haliaeetinae, Buteoninae) and U-shaped (Perninae, most Gypaetinae, Aegypiinae, Circaetinae, Haliaeetinae, Buteoninae). The distal end of the attachment scar for the spina fibulae (Fig. 8, SFA) is well proximal to the condylus lateralis (most Perninae, Aquilinae, most Haliaeetinae, Buteoninae). The epicondylus medialis (Fig. 8, EM) is projecting medially of the condylus medialis in cranial aspect (Perninae, Gypaetinae, Aegypiinae, Circaetinae, most Haliaeetinae, Buteoninae). The sulcus extensorius (Fig. 8, SE) is deep (Perninae, Gypaetinae, Aegypiinae, some Circaetinae, Aquilinae, Haliaeetinae, Buteoninae).
The condylus medialis is distinctly more robust than the condylus lateralis and in cranial aspect widens distally (Haliaeetinae) because of the impression of crus mediale ligamentum meniscotibialis deeply excavates the base of the proximal end of the condyle (Haliaeetinae). The impression of crus laterale ligamentum meniscotibialis is a deep wide scar alongside, but not excavating, the base of the condylus lateralis (Perninae, Gypaetinae, Aquilinae, Buteoninae). In cranial aspect, the notch distally between the condyles is broadly U-shaped (most accipitrids excluding Aquilinae).
Tarsometatarsus (Fig. 9)
Dynatoaetus gaffae (SAMA P.59525) right tarsometatarsus from Mairs Cave in plantar (A), dorsal (B), proximal (C) and distal (D) aspect, fragment of right distal tarsometatarsus from Victoria Fossil Cave (SAMA P.28008) in dorsal (E) aspect, along with the os metatarsale and first phalanx of pedal digit I (F), first and second phalanges of digit II (G), first phalanx of digit IV (H), and three ungual phalanges (SAMA P.59525, I; SAMA P.19157, J; SAMA P.17139, K) from Mairs Cave. CM crista medianoplantaris, FID fossa infracotylaris dorsalis, FLC fovea ligamentum collateralis, FPM fossa parahypotarsalis medialis, FVPM foramen vasculare proximale medialis, HC hypotarsal crista, ILCL impressio ligamentum collateralis lateralis, IRE impressiones retinaculi extensorii attachment scars, MPT medial plantar tuberculum, OM os metatarsale, PI.1 phalanx 1 digit 1, P2.1 phalanx 1 digit 2, P2.2 phalanx 2 digit 2, PIV.1 phalanx 1, digit 4, SF sulcus flexorius, TMII trochlea metatarsi II, TMIII trochlea metatarsi III, TMIV trochlea metatarsi IV, TMTC tuberositas m. tibialis cranialis. Scale bars are 10 mm
The Mairs Cave holotype specimen is almost complete, lacking only a small part of the proximal medial shaft margin and the plantar parts of the cristae hypotarsi. Only trochleae metatarsorum II and III are preserved in SAMA P.28008, limiting comparisons, but it is identical to the Mairs Cave specimen. The following features are observed:
The hypotarsal cristae (Fig. 9, HC) are widely separated (all Accipitridae excluding Gypaetinae, most Aegypiinae). Consequently, the fossa parahypotarsalis medialis (Fig. 9, FPM) is narrow, allowing for damage to its medial margin (8 mm, or 25% of proximal width) (most Accipitridae except Aquilinae). The fossa parahypotarsalis medialis merges with the sulcus flexorius (Fig. 9, SF) in the proximal quarter of the shaft (most Accipitridae except Haliaeetinae) and together make a deep sulcus such that the shaft dorsal to them is very thin (all accipitrids except Gypaetinae, Aegypiinae). The foramen vasculare proximale medialis (Fig. 9, FVPM) is directly adjacent to the distal extremity of the crista medianoplantaris (Fig. 9, CM) (some Perninae, most Circaetinae, some Aegypiinae, some Aquilinae, most Haliaeetinae, Buteoninae). The attachment scars for the impressiones retinaculi extensorii (Fig. 9, IRE) are prominent (most Accipitridae except for Aegypiinae and some Gypaetinae), with a deepened depression between them. The tuberositas m. tibialis cranialis (Fig. 9, TMTC) is 11 mm long, a single oval-shaped tuberosity (most Accipitridae except Aviceda (Perninae), Pithecophaga (Circaetinae), Haliaeetus (Haliaeetinae), and Buteoninae) and projects moderately cranially from the shaft in lateral aspect (most Accipitridae except Gypaetinae, some Aegypiinae). The tuberositas m. tibialis cranialis is positioned almost directly distal to the foramina vascularis proximalia (most Perninae, Gypaetinae, Aegypiinae, Circaetinae, Harpiinae, Haliaeetinae, Buteoninae). The fossa infracotylaris dorsalis (Fig. 9, FID) is moderately deep (most Gypaetinae, some Circaetinae, Aquilinae, Buteoninae) and is close (4 mm) to them. The impressio ligamentum collateralis lateralis (Fig. 9, ILCL) is ~ 15 mm long and laterally prominent (similar in some Perninae, most Circaetinae, some Aegypiinae, most Haliaeetinae). The medial shaft margin is strongly compressed (most Accipitridae), and forms a thin, sharp ridge over the proximal half of length.
Trochlea metatarsi II (Fig. 9, TMII) is the most robust trochlea and extends slightly distal to trochlea metatarsi III (Fig. 9, TMIII) (some Perninae, Haliaeetinae). Trochlea metatarsi IV (Fig. 9, TMIV) is the narrowest and distally shortest trochlea (most Accipitridae). Only trochlea metatarsi III is grooved (most Accipitridae). The trochleae flare equally medially and laterally. The flange on trochlea metatarsi II is robust, oriented medioplantarly and is moderately long (Circaetinae, Aquilinae, Haliaeetinae, Buteoninae). The flange on trochlea metatarsi IV projects strongly plantarly (most Accipitridae excluding Aegypiinae). The fovea ligamentum collateralis (Fig. 9, FLC) is large and deep (Circaetinae, Aquilinae, Buteoninae). The foramen vasculare distale (Fig. 9, FVD) is elongate and large (6 mm long). Fossa metatarsi I is large, entirely on the plantar facies but its medial rim impinges on the medial shaft profile (most Accipitridae except Gyps (Aegypiinae), some Perninae).
Pedal phalanges (Fig. 9)
The right metatarsal 1, LI.1, RII.1. RII.2, RVI.1, and 3 ungual phalanges are preserved. The following features can be observed:
The metatarsal (Fig. 9, OM) is heavily eroded around its margins, making some features difficult to observe. The lateral margin leading to the trochlea metatarsi I (Fig. 9, TMI) has a largely straight profile, only curving outward laterally directly proximal to the trochlea. The main feature is that all phalanges are relatively short and very robust, such that lengths do not differ much from those of a female Aquila audax and they are very much stouter.
The phalanx I.1 (Fig. 9, PI.1) is missing the distal trochlea beyond its plantar base, nevertheless it was likely not longer than that of Aquila audax, having a very much shorter mid-section. The medial plantar tuberculum (Fig. 8, MPT) is very large and robust.
The phalanx II.1 (Fig. 9, PII.1) is not fused with II.2 (all accipitrids excluding Haliaeetinae). Concomitant with its robust nature, the trochlea articularis of II.1 is broad and deep for reception of the tuberculum extensorium of II.2.
The phalanx II.2 (Fig. 9, PII.2) is robust and relatively short, being roughly twice the length of the II.1, and it tightly interlocks with it precluding rotation at the joint. The corpus phalangis is swollen both medially and laterally just proximal to the foveae ligamenti collaterales, much more so than in Aquila audax.
The phalanx IV.1 (Fig. 9, PIV.1) has a moderate amount of lateral expansion. The foveae ligamenti collaterales distally are shallow and poorly defined. In side view, the dorsal surface is deeply concave because of a much enlarged dorsal margin to the proximal articular surface. In plantar view, a robust tuberculum flexorium forms a ridge extending plantarly along the length of the lateral side of the digit. Medially, a robust and short tuberculum flexorium is proximally expanded.
Ungual phalanges (Fig. 9)
The three ungual phalanges are each nearly perfectly preserved, missing only the very tip of the distal end. Due to a lack of associated pedal phalanges, it is uncertain to which digits these unguals belong, but ungual phalanges SAMA P.59525 and SAMA P.19157 seem to be a pair based on similar morphology. All specimens are notably larger than ungual phalanges one and two in the observed specimens of Aquila audax. Additionally, the following features are observed: The height of the articular facet is greater than its width. In P.17139, the width of the tuberculum flexorium is less than its height thanks to deepened depressions distal of the foramina, and the foramina on the sides of the tuberculum flexorium are distinct and deep. The width of the tuberculum flexorium in P.19157 is roughly equal to its height.
Phylogenetic analyses
Parsimony analysis of morphological and molecular data produced three most parsimonious trees (MPTs) of length of 1835, the strict consensus of which is shown in SI.8.
Dynatoaetus gaffae was weakly supported as sister to the living Aegypiinae (51.5%). This weak support also extends to support for the Circaetinae clade (48.4%) and the Aegypiinae–Circaetinae clade (54.8%). The divergence of the Aegypiinae–Circaetinae from all other accipitrids has moderate support (71.4%), as does the divergence of the Harpiinae–Aquilinae (63.4%) and the divergence of Harpiinae from Aquilinae (67.2%).
The clade comprising D. gaffae and Aegypiinae was supported by nine character states that optimised unambiguously on that branch (character number in brackets): the alignment of the lateral edges of the processus paroccipitalis of the neurocranium being roughly parallel (29: 1 → 0); the foramen magnum of the neurocranium being positioned on the caudoventral plane (30: 2 → 1); the crista medialis carinae does not abut with the spina externa on the sternum (71: 0 → 1); the apex carinae is positioned caudal to the sulcus sellaris medialis in the dorsoventral axis on the sternum (75: 1 → 0); the depth of the carina is less than the depth of the sternum basin (89: 1 → 2); the fossa m. brachialis of the numerus is narrowly separated from the dorsal facies of the shaft (151: 1 → 2); the carpometacarpus lacks a deepened sulcus separating the processus pisiformis and processus extensorius (180: 1 → 0); the ridge bordering the distal end of the sulcus tendinous on the carpometacarpus has a low profile (185: 1 → 0); the medial shaft margin adjacent to the mid-length fossa parahypotarsalis medialis is dorsoplantarly compressed but slightly thickened (263: 2 → 1). Only one of these optimisation-unambiguous characters, 71, was also unique and unreversed (CI = 1.00). Of these character states, only those for 29, 71, 75, 89, 151, 185 and 263 are unique or mostly unique to the Aegypiinae in accipitrids (29, 75, 89, and 151 are seen in some Gypaetinae, 263 seen in a small number of Gypaetinae and Perninae).
The clade comprising D. gaffae, Aegypiinae and Circaetinae was diagnosed by three unambiguous characters: the fossa sternocoracoidei on the sternum extending to the fourth processus costalis (80: 3 → 2); the os metacarpale majus on the carpometacarpus has weak ventral projection (186: 1 → 0); the first phalanx of pedal digit I is equal to slightly more robust than most other phalanges (285: 2 → 1).
Dynatoaetus gaffae was excluded from the clade comprising Harpiinae, Aquilinae, Haliaeetinae, Buteoninae and Accipitrinae by lacking four unambiguous characters: the processus lateralis of the mandible forms a long lateral projection (54: 1 → 2); presence of foramen pneumaticum in dorsal facies of sternum basin (91: 0 → 1); the linea intermuscularis cranialis of the femur is positioned medially on the shaft (209: 0 → 1).
When Cryptogyps lacertosus was added to the phylogeny (Fig. 10), neither the position of D. gaffae nor support for the fossil-Aegypiinae clade changed notably (51.2%). The characters supporting this clade were unchanged from the previous analysis. The clade comprising C. lacertosus and Aegypiinae had good support (78.5%), but support for Aegypiinae–Circaetinae (50%) and the Circaetinae clade (44.4%) was low. The divergence of the fossils-Aegypiinae–Circaetinae clade dropped (65.2%), as did the divergence of the Harpiinae–Aquilinae from all other Accipitridae (58.8%) and the Harpiinae clade to a very small degree (64.2%). A morphology-only parsimony analysis tree using the same taxa is presented in SI.8. In this tree, Dynatoaetus resolves as the sister taxon of a group comprising both Aegypiinae (including Cryptogyps) and Gypaetinae, though DNA studies strongly suggest that the morphological similarities between Aegypiinae and Gypaetinae are due to convergence. Similarly, incorrect associations were seen in some other morphologically similar taxa; the circaetine Pithecophaga jefferyi and the harpiine Harpia harpyja were sister to each other and nested within the Aquilinae, while Chondrohierax uncinatus and Hamirostra melanosternon were separated from other Perninae. As the aim of the phylogenetic analyses was to place the fossils within the most widely accepted phylogeny of living taxa, this justifies our use of molecular data to effectively constrain the relationships of living taxa.
The Bayesian analysis (Fig. 11) of the data matrix including both Dynatoaetus and Cryptogyps produced trees mostly concordant with the parsimony analysis. Dynatoaetus gaffae was positioned basal to all the Aegypiinae, but with low support (pp [posterior probability] = 0.49). Cryptogyps lacertosus was separated from other Aegypiinae into its own branch, but with very weak support (0.35). Support for the Circaetinae–Aegypiinae clade (including D. gaffae), and the grouping of this clade with all other Accipitridae, was 0.52. Both fossil species were strongly excluded from the Aquilinae–Harpiinae–Accipitrinae–Haliaeetinae–Buteoninae clade, which had a pp of 1.0.
Discussion
Dynatoaetus gaffae is a new genus and species of large accipitrid raptor from the Australian Pleistocene, based on relatively complete material comprising 32 bones of a partial skeleton and 4 other referred fossil bones from three other sites. It is up to twice the mass of the largest living Australian eagle, the Wedge-Tailed Eagle Aquila audax, and of all other fossil eagles known from the continent.
Phylogenetic affinities
Dynatoaetus gaffae exhibits a mixture of features found across species of Perninae, Gypaetinae, Circaetinae and Aegypiinae. These are likely a combination of phylogenetic and functional features, making assessment of the precise taxonomic position of the fossil difficult using only comparative morphology. All phylogenetic analyses (morphology only, parsimony and bayesian analyses of mixed molecular and morphology data) found D. gaffae to resolve within the clade of Circaetinae + Aegypiinae as sister to Aegypiinae. The Circaetinae include a large raptorial species (Pithecophaga jefferyi) in the Philippines.
Dynatoaetus gaffae can be distinguished from species of Circaetinae, the sister clade to Aegypiinae (see Mindell et al. 2018), found geographically close to Australia (notably Spilornis cheela and Pithecophaga jefferyi) by the following features (circaetine state in brackets): the processus postorbitalis ends dorsal to the processus zygomaticus (extends level), the foramen magnum is positioned caudoventrally on the caudal end of the skull (completely ventral), prominent tubercula are present on the caudal section of the area muscularis aspera (absent); on the sternum, the processus labra internum prominently project cranially (absent in Pithecophaga, small projection in all others), the crista medialis terminates before the base of the spina externa (extends to spina externa), the apex carinae tip is positioned caudal to the sulci articulares coracoidei (adjacent to sulci), there are thought to have been six processus costales (seven in Pithecophaga + Spilornis); in the scapula, the tuberculum coracoideum barely projects cranially (prominent projection), a small foramen is present in the ventral facies of the cranial end (absent), the facies articularis humeralis is continuous with the ventral shaft margin (facies projects out from shaft); in the carpometacarpus, the facies alularis digiti alulae is continuous with the shaft (separated from shaft by a notch), a shallow sulcus separating the processus pisiformis and processus extensorius (deep sulcus); in the ulna, the tuberculum carpale is flattened cranially (moderately projecting); in the femur, the crista supracondylaris medialis is prominent (flat), and the impressio lig. collateralis lateralis spans roughly two-thirds the caudo-cranial depth of the condylus lateralis (a third of craniocaudal depth or less); in the tibiotarsus, the condylus medialis does not expand medially past the shaft margin (extends further medially); in the tarsometatarsus, the foramen vasculare proximalis medialis is positioned lateral to the crista medioplantaris (positioned medial to crista), there is a deep depression between the impressiones retinaculi extensorii (no depression).
Dynatoaetus gaffae differs from living species of Aegypiinae by the following features (aegypiine state in brackets): a lack of pneumatism in the distal wing bones and sternum. In the neurocranium, the lamina parasphenoidalis is unfused (fused), and there are prominent tubercles present on the area muscularis aspera (absent). In the sternum, the sulci articularis coracoidei overlap (do not overlap); the processus labrum internum are present and quite prominent (absent or greatly reduced). In the scapula, there is no foramen/pneumatisation present in the cranial region of the acromion (present); a foramen is present in the medial facies of the cranial end (absent); the facies articularis humeralis is continuous with the shaft margin (ends with a ventrally offset notch); the depth of the corpus scapulae increases caudally (remains largely the same). In the tarsometatarsus, the merged sulcus flexorius and fossa parahypotarsalis medialis forms a deepened sulcus (shallow); prominent attachment scars for the impressions retinaculi extensorii (greatly reduced/absent); the fossa infracotylaris dorsalis is deep (shallow); the flange on the trochlea metatarsi II prominently projects medioplantarly (little projection); the flange on trochlea metatarsi II prominently projects plantarly (little projection); the fovea ligamentum collateralis is deep (shallow). Furthermore, Dynatoaetus gaffae has traits quite unlike the living crown aegypiines, such as large raptorial ungual phalanges and a tarsometatarsus with typically eagle-like proportions and features.
The fossil differs from any living species of Gypaetinae (which associated with aegypiines in the morphology-only parsimony analysis) by the following features (gypaetine state in brackets): in the sternum, the spina externa is narrower than the apex carinae in cranial view (thicker or equal thickness), the crista medialis carinae terminating proximal to the sulci coracoidei (terminating adjacent); in the humerus, the proximal and distal heads of the m. pronator profundus are large and deep (small and shallow). In the tarsometatarsus, the foramina vascularia proximalia is positioned lateral to the crista medialis hypotarsi (positioned medial). The fossil is much larger than the small gypaetine vultures Neophron percnopterus and Gypohierax angolensis, and is also notably larger than Gypaetus barbatus, a species which is slightly larger than Aquila audax.
These differences reveal the highly distinct nature of Dynatoaetus gaffae and warrants the establishment of a new genus for the taxon.
Ecology
The known distribution of Dynatoaetus gaffae is currently limited to the southern parts of the continent (see Fig. 1) from the Cooper Creek of the Lake Eyre Basin, Mairs Cave, Wellington Caves and Naracoorte Caves region, in central Australia, mid-north South Australia, central eastern NSW and southeast South Australia, respectively. However, this may reflect abundance of fossil sites in these regions and the expected rarity of a top predator. A broader distribution over the continent would not be unusual, given the range of many modern Australian raptors (see Simpson and Day 2010).
Despite its much larger size, the pedal phalanges of Dynatoaetus gaffae do not seem to have been much longer than that of a large Aquila audax, suggesting similar foot span in both species. However, the phalanges are comparatively much more robust in D. gaffae, which could indicate a more powerful grip. This kind of morphology can be observed in accipitrid species such as Stephanoaetus coronatus, which is known to prey upon monkeys and small antelope (Sanders et al. 2003); Harpia harpyja, which primarily hunts sloths and monkeys but will also rarely take terrestrial animals like the collared peccary Tayassu tajacu (see Miranda 2018); and Hieraaetus moorei, which is known to have preyed upon large species of Dinornithiformes (Brathwaite 1992; Worthy and Holdaway 2002). This indicates that D. gaffae could also have been adapted for hunting relatively large prey, using its strength to maintain its grip on the struggling animal. The late Pleistocene Australian fauna included species like the sthenurine kangaroos, larger macropodine kangaroos, large snakes, giant megapodes, the flightless Genyornis, and the diprotodontids (Wroe et al. 2013), the young and sickly of which D. gaffae may have preyed upon.
While D. gaffae has a predatory morphology, it is likely that it also scavenged frequently upon the carcasses of megafaunal marsupials, as do many modern predatory eagles (see Kane et al. 2014; Margalida et al. 2017). It would have faced competition from specialist avian scavengers such as Cryptogyps lacertosus but the size of D. gaffae would have given it an advantage against this smaller species. This is observed in modern avian scavenging guilds, where the largest, most belligerent birds tend to maintain dominance around carcasses (Moreno-Opo et al. 2020). No taphonomic traces on fossil megafaunal material have been referred to a large raptor to date but this likely reflects bias in consideration of possible predators and scavengers.
Comparisons with other large extinct accipitrids
The size of Dynatoaetus gaffae is quite large compared to most living eagles, but it is still smaller than the largest known individuals of two other distantly related extinct species: Hieraaetus moorei from New Zealand, and Gigantohierax suarezi Arredondo and Arredondo (2002) from Cuba. Hieraaetus moorei is known to be closely related to the living Little Eagle Hieraaetus morphnoides of the Aquilinae (Bunce et al. 2005; Knapp et al. 2019), while G. suarezi was referred to the Buteoninae by Arredondo and Arredondo (2002), though there was little justification given for this conclusion. The Philippines and South America have the large living species Pithecophaga jefferyi (Circaetinae) and Harpia harpyja (Harpiinae) respectively. Therefore, at least five accipitrid clades have produced extremely large predators in their respective ecological systems.
Both H. moorei and G. suarezi lived on islands that lacked large mammalian or reptilian predators and had large-bodied prey. Dynatoaetus gaffae, in contrast, lived on a continent where it may have had to compete for prey, or at least interact, with competitor species like Thylacoleo carnifex (marsupial apex predator) and Varanus priscus (giant monitor lizard). This would almost certainly have impacted D. gaffae in its behaviour, body size limit, and niche in ways that would not be present in the island-dwelling eagles. The large body size of D. gaffae is most likely due to phylogenetic history (most aegypiines are rather large, even for raptors) rather than being the product of insular gigantism.
Dynatoaetus gaffae was also similar in size to some extinct Pleistocene aegypiines. The vulture Gyps melitensis Lydekker (1890), from the Pleistocene of Malta, would have been of similar or slightly larger size than D. gaffae (see Louchart 2002; Marco 2007; Manegold and Hutterer 2021). Similarly, Aegypius jinniushanensis and an unnamed species of Torgos from the Pleistocene of China may also have been similar in size to D. gaffae based on the size of known cranial material, though the neurocranium of D. gaffae is narrower and taller than these species (Zhang et al. 2012). However, there would naturally be differences in wing and leg proportions between a scavenger and an active hunter, which makes assessing overall size differences difficult.
Implications for modern fauna
Dynatoaetus gaffae was the largest predatory bird in Australia during the Pleistocene and the largest known from the Australian fossil record. Very few, if any, Australian raptors could have directly competed with it. The taxonomic affinities of D. gaffae, therefore, challenge current understandings of how eagles in the genus Aquila emerged as the top Australian avian predator in modern ecosystems. The Wedge-Tailed Eagle Aquila audax is part of a clade with species in Africa and Europe that diverged a little over two million years ago, and the diversification of Aquila and related genera occurred around four million years ago (Mindell et al. 2018).
The fossil record of Aquila audax is quite limited, with most fossils known from late Pleistocene deposits (Baird 1991, see p 829), and a few from mid Pleistocene deposits from Victoria Fossil Cave (unpublished data) where fossil material of D. gaffae has also been found. This indicates that there was overlap between the two species, which in turn suggests there would have been niche separation. The late Miocene Aquila bullockensis Gaff and Boles (2010), from the 11–9 Ma Camfield Beds is the oldest putative example of the genus from Australia, and possibly the world. This would suggest Aquila has a long history in Australia. However, this proposed antiquity strongly conflicts with the molecular dates, which suggest that the broad clade including Aquila plus related genera is less than 5 Ma old (Mindell et al. 2018). Furthermore, A. bullockensis was referred to Aquila based solely on the description of a distal humerus. This holotype of A. bullockensis differs from A. audax in the reduced distal projection of the processus flexorius, limited cranial projection and lack of proximal narrowing in the tuberculum supracondylare ventrale, minimal convexity between the processus supracondylaris dorsale and epicondylus dorsalis, and the dorsal insertion of the m. extensor radii being offset from the processus supracondylare dorsale (Mather et al. 2021). It is entirely possible that A. bullockensis would have its generic referral changed if more material was considered, and so is not strong evidence for the presence of Aquila during the Miocene. In combination with the presence of a clade of stem-aegypiine accipitrids that occupied hunting and scavenging niches, it seems likely that Aquila is a recent arrival to Australia.
Aquila audax is presently widespread across inland Australia and is the top avian predator of most terrestrial ecosystems. The prey of A. audax typically ranges in size from rabbits to small kangaroos, and it frequently scavenges from carcasses (Brooker and Ridpath 1980; Olsen et al. 2010). It is unlikely that this generalist habit would have been possible when accipitrid species such as the giant predatory D. gaffae and the vulturine Cryptogyps lacertosus were still alive to provide competition. In modern ecosystems where multiple species of raptors are present, the partitioning of resources such as food and nesting sites allows such coexistence to occur (Krüger 2002; Giovanni et al. 2007; Olsen et al. 2010; Treinys et al. 2011; Whitfield et al. 2013). It is, therefore, possible that until the late Pleistocene, Aquila audax was limited to hunting a smaller range of prey, scavenged less frequently, and may have been more restricted in its range due to interspecific competition. The extinction of the Australian megafauna would have resulted in the subsequent extinction of scavengers like C. lacertosus and large predators like D. gaffae (see Galetti et al. 2018), leaving the surviving generalist A. audax free to exploit their former niches.
Data availability
Data was collected from specimens in the institutions listed within the paper. All data for this research is available either within the paper itself or the accompanying SI, and may be used with the proper citations.
References
Alley NF (1998) Cainozoic stratigraphy, palaeoenvironments and geological evolution of the Lake Eyre Basin. Palaeogeogr Palaeoclimatol Palaeoecol 144:239–263
Arnold LJ, Demuro M, Power R, Priya DM, Guilarte V, Weij R, Woodhead J, White L, Bourne S, Reed EH (2022) Examining sediment infill dynamics at Naracoorte cave megafauna sites using multiple luminescence dating signals. Quat Geol 70:2. https://doi.org/10.1016/j.quageo.2022.101301
Arredondo O (2002) Arredondo C (2002) [1999] Nuevos género y especie de ave fósil (Falconiformes: Accipitridae) del Cuaternario de Cuba. Poeyana 470–475(1999):9–14
Ayliffe LK, Marianelli PC, Moriarty KC, Wells RT, McCulloch MY, Mortimer GE, Hellstrom JC (1998) 500 Ka precipitation record from southeastern Australia: evidence for interglacial relative aridity. Geology 26:147–150
Baird RF, Rich PV, van Tets GF (1991) Localities yielding avian assemblages of Quaternary age in Australia. Appendix II, in Baird (1991), in: Vertebrate Palaeontology of Australasia, Vickers-Rich P, Monaghan JM, Baird RF, Rich TH (eds). Pioneer Design Studio Ltd, Lilydale, & Monash University Publications Committee, Melbourne, pp 850–870.
Baird RF (1991) Avian fossils from the Quaternary of Australia. Chapter 21 in: Vickers-Rich P, Monaghan JM, Baird RF, Rich TH (eds) Vertebrate Palaeontology of Australasia. Pioneer Design Studio Ltd, Lilydale, & Monash University Publications Committee, Melbourne: pp 809–870
Baumel JJ, Witmer LM (1993) Osteologia. 45–132. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC (eds) Handbook of avian anatomy: nomina anatomica avium, 2nd edition. Publications of the Nuttall Ornithological Club 23. Nuttall Ornithological Club Cambridge, MA.
Bell PR (2004) Mammal bones and talus cones: stratigraphy, palaeoecology and palaeontology of Phosphate Mine East, Wellington Caves, NSW. Unpublished BSc Honours thesis, Macquarie University, Sydney
Boles WE (1993) Pengana robertbolesi, a peculiar bird of prey from the Tertiary of Riversleigh, northwestern Queensland, Australia. Alcheringa 17:19–25
Boles WE (2006) The avian fossil record of Australia: an overview. In: Merrick JR, Archer M, Hickey GM, Lee MSY (eds) Evolution and biogeography of Australasian vertebrates. Auscipub Pty Ltd, Oatlands, pp 387–411
Brathwaite DH (1992) Notes on the weight, flying ability, habitat, and prey of Haast’s eagle (Harpagornis moorei). Notornis 39:239–247
Brooker MG, Ridpath MG (1980) The diet of the Wedge-Tailed Eagle, Aquila audax, in Western Australia. Aust Wildlife Res 7:433–452
Bunce M, Szulkin M, Lerner HRL, Barnes I, Shapiro B, Cooper A, Holdaway RN (2005) Ancient DNA provides new evolutionary insights into the evolutionary history of New Zealand’s extinct giant eagle. PLoS Biol 3:9. https://doi.org/10.1371/journal.pbio.0030009
Burleigh JG, Kimball RT, Braun ED (2015) Building the avian tree of life using a large-scale, sparse supermatrix. Mol Phylogenet Evol 84:53–63
Callen RA, Nanson GC (1992) Formation and age of dunes in the Lake Eyre depocentres. Geol Rundsch 81:589–593. https://doi.org/10.1007/BF01828619
Campbell V, Lapointe FJ (2009) The use and validity of composite taxa in phylogenetic analysis. Syst Biol 58:560–572
Dawson L (1985) Marsupial fossils from Wellington Caves, New South Wales; the historic and scientific significance of the collections in the Australian Museum. Rec Aust Mus 37:55–69
Dawson L, Muirhead J, Wroe S (1999) The Big Sink Local Fauna: a lower Pliocene mammalian fauna from the Wellington Caves complex, Wellington, New South Wales. Records Western Aust Museum 57:265–290
Dawson L (1995) Biostratigraphy and biochronology of sediments from the Bone Cave, Wellington Caves, NSW, based on vertebrate fossil remains. Abstract, Quaternary Symposium. The Linnean Society of New South Wales, Wellington Caves
de Vis CW (1892) Residue of the extinct birds of Queensland as yet detected. Proc Linnean Soc NSW 6:437–456
de Vis CW (1905) A contribution to the knowledge of the extinct avifauna of Australia. Ann Queensl Museum 6:3–25
Dickinson EC, Remsen JV (eds) (2013) The Howard & Moore complete checklist of birds of the world. Aves Press, Eastbourne
Elzanowski A, Stidham TA (2010) Morphology of the quadrate in the Eocene anseriform Presbyornis and extant galloanserine birds. J Morphol 271:305–323
Elzanowski A, Zelenkov NV (2015) A primitive heron (Aves: Ardeidae) from the Miocene of Central Asia. J Ornithol 156:837–846
Fischer MJ (1997) Speleothem chronology and its implications for biochronology, Wellington Caves, New South Wales. Abstract, CAVEPS, July 1997
Fraser RA, Wells RT (2006) Palaeontological excavation and taphonomic investigation of the late Pleistocene fossil deposit in Grant Hall, Victoria Fossil Cave, Naracoorte, South Australia. Alcheringa Aust J Palaeontol 30:147–161
Gaff P (2002) The fossil history of the family Accipitridae in Australia. Unpublished MSc thesis, Monash University, Victoria, Australia
Gaff P, Boles WE (2010) A new eagle (Aves: Accipitridae) from the mid Miocene bullock creek fauna of northern Australia. Rec Aust Mus 62:71–76
Galetti M, Moleón M, Fordano P, Pires MM, Guimarães PR Jr, Pape T, Nichols E, Hansen D, Olesen JM, Munk M, de Mattos JS, Schweiger AH, Owen-Smith N, Johnson CN, Marquis RJ, Svenning J (2018) Ecological and evolutionary legacy of megafaunal extinctions. Biol Rev 93:845–862
Gill F, Donsker D, Rasmussen P (eds) (2020) IOC World Bird List (v10.2). https://doi.org/10.14344/IOC.ML.10.2
Giovanni MD, Boal CW, Whitlaw HA (2007) Prey use and provisioning rates of breeding Ferruginous and Swainson’s hawks on the southern Great Plains, USA. Wilson J Ornithol 119:558–569
Gould-Whaley C, Drysdale R, May J-H, Hellstrom J, Cheng H, Woodhead J, Greig A, Corrick E, Cohen T (2021) Subaqueous speleothems from the Flinders Ranges, South Australia, as palaeohydrological archives for the arid zone. EGU General Assembly 2021, online, 19–30 Apr 2021, EGU21-16120. https://doi.org/10.5194/egusphere-egu21-16120, 2021.
Grün R, Moriarty K, Wells R (2001) Electron spin resonance dating of the fossil deposits in the Naracoorte Caves, South Australia. J Quat Sci 16:49–59
Haast J (1872) Notes on Harpagornis moorei, an extinct gigantic bird of prey, containing description of femur, ungual phalanges, and rib. Trans Proc New Zealand Inst 4:192–196
Hocknull SA, Lewis R, Arnold LJ, Pietsch T, Joannes-Boyau R, Price GJ, Moss P, Wood R, Dosseto A, Louys J, Olley J, Lawrence RA (2020) Extinction of eastern Sahul megafauna coincides with sustained environmental deterioration. Nat Commun 11:1–14
Holdaway RN (1991) Systematics and palaeobiology of Haast’s eagle (Harpagornis moorei Haast, 1872) (Aves: Accipitridae). Unpublished PhD thesis. Department of Zoology, University of Canterbury, Christchurch, New Zealand
Kane A, Jackson AL, Ogada DL, Monadjem A, McNally L (2014) Vultures acquire information on carcass location from scavenging eagles. Proc R Soc B 281:20141072
Knapp M, Thomas JE, Haile J, Prost S, Ho SYW, Dussex N, Cameron-Christie S, Kardailsky O, Barnett R, Bunce M, Gilbert MTP, Scofield RP (2019) Mitogenomic evidence of close relationships between New Zealand’s extinct giant raptors and small-sized Australian sister-taxa. Mol Phylogenet Evol 134:122–128
Kraehenbuehl P, Lawrence RE, Flavel S (1997) Caves of the Flinders Ranges. Cave exploration group of South Australia Occasional Paper 9:57
Krüger O (2002) Analysis of nest occupancy and nest reproduction in two sympatric species of raptors: common buzzard Buteo buteo and goshawk Accipiter gentilis. Ecography 25:523–532
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B (2017) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol 34:772–773
Liddle NR, McDowell MC, Prideaux GJ (2018) Insights into the pre-European mammal fauna of the southern Flinders Ranges, South Australia. Aust Mammal 40:262–268
Louchart A (2002) Les oiseaux du Pléistocène de Corse, et de quelques localités sardes – écologie, évolution, biogéographie et extinctions. Documents Des Laboratoires De Géologie De Lyon 155:1–287
Lydekker R (1890) On the remains of some large extinct birds from the cavern-deposits of Malta. Proc Zool Soc Lond 1890:403–411
Macken AC, Reed EH (2013) Late quaternary small mammal faunas of the Naracoorte Caves World Heritage Area. Trans R Soc S Aust 137:53–67
Macken AC, Jankowski NR, Price GJ, Bestland EA, Reed EH, Prideaux GJ, Roberts RG (2011) Application of sedimentary and chronological analyses to refine the depositional context of a Late Pleistocene vertebrate deposit, Naracoorte, South Australia. Quatern Sci Rev 30:2690–2702
Macken AC, McDowell MC, Bartholomeusz DN, Reed EH (2013) Chronology and stratigraphy of the Wet Cave fossil deposit, Naracoorte, and relationship to paleoclimatic conditions of the Last Glacial Cycle in south-eastern Australia. Aust J Earth Sci 60:271–281
Magee JW, Bowler JM, Miller GH, Williams DLG (1995) Stratigraphy, sedimentology, chronology and palaeohydrology of Quaternary lacustrine deposits at Madigan Gulf, Lake Eyre, South Australia. Palaeogeogr Palaeoclimatol Palaeoecol 113:3–42
Manegold A, Hutterer R (2021) First substantial evidence for Old World vultures (Aegypiinae, Accipitridae) from the early Palaeolithic and Iberomaurusian of Morocco. PalZ 95:503–514
Marco AS (2007) New occurrences of the extinct vulture Gyps melitensis (Falconiformes, Aves) and a reappraisal of the paleospecies. J Vertebr Palaeontol 27:1057–1061
Margalida A, Colomer M, Sánchez R, Sánchez FJ, Oria J, Sánchez LM (2017) Behavioural evidence of hunting and foraging techniques by a top predator suggests the importance of scavenging for preadults. Ecol Evol 7:4192–4199
Maroulis JC, Nanson GC, Price DM, Pietsch T (2007) Aeolian-fluvial interaction and climate change: source-bordering dune development over the past ~100 ka on Cooper Creek, central Australia. Quatern Sci Rev 26:386–404
Mather EK, Lee MSY, Camens AB, Worthy TH (2021) An exceptional partial skeleton of a new basal raptor (Aves: Accipitridae) from the late Oligocene Namba formation, South Australia. Hist Biol 34:1175–1207. https://doi.org/10.1080/08912963.2021.1966777
Mather EK, Lee MSY, Worthy TH (2022) A new look at an old Australian raptor places “Taphaetus” lacertosus de Vis 1905 in the Old World vultures (Accipitridae: Aegypiinae). Zootaxa 5168:1–23
Mather EK (2021) Taxonomy of fossil eagles and vultures (Aves, Accipitridae) from Australia. Unpublished PhD thesis, Flinders University, South Australia, Australia.
Matsuoka H, Seoka R (2021) Myology and osteology of the Whooper Swan Cygnus cygnus (Aves: Anatidae) Part 3. Muscles attached to the pelvis, femur, tibiotarsus, tarsometatarsus and phalanx. Bull Gunma Museum of Nat Hist 25:1–18
Mayr G (2014) Comparative morphology of the radial carpal bone of neornithine birds and the phylogenetic significance of character variation. Zoomorphology 133:425–434
Megirian D, Prideaux GJ, Murray PF, Smit N (2010) An Australian land mammal age biochronological scheme. Paleobiology 36:658–671
Meyer AB (1897) Abbildungen von Vogelskeletten, vol 2. R. Friedländer & Sohn, Berlin
Migotto R (2013) Phylogeny of Accipitridae (Aves: Accipitriformes) based on osteological characters. PhD Dissertation, Universidade de São Paulo, Insituto de Biociências, Departmento de Zoologia. https://teses.usp.br/teses/disponiveis/41/41133/tde-08102013-105334/en.php
Mindell DP, Fuchs J, Johnson JA (2018) Phylogeny, taxonomy, and geographic diversity of diurnal raptors: Falconiformes, Accipitriformes, and Cathartiformes. In: Sarasola JH, Grande J, Negro J (eds) Birds of prey. Springer, Cham, pp 3–32
Miranda EBP (2018) Prey composition of Harpy Eagles (Harpia harpyja) in Raleighvallen, Suriname. Trop Conserv Sci 11:1–8
Moreno-Opo R, Trujillano A, Margalida A (2020) Larger size and older age confer competitive advantage: dominance hierarchy within European vulture guild. Sci Rep 10(2430):1–12
Nagy J, Tökölyi J (2014) Phylogeny, historical biogeography and the evolution of migration in accipitrid birds of prey (Aves: Accipitriformes). Ornis Hungarica 22:15–35
Nanson GC, Price DM, Short SA (1992) Wetting and drying of Australia over the past 300 ka. Geology 20:791–794. https://doi.org/10.1130/0091-7613(1992)020%3c0791:WADOAO%3e2.3.CO;2
Nipperess D (2002) The Koppa’s Pool local fauna, an early Pliocene vertebrate assemblage from the Wellington Caves complex, Australia
Olsen J, Judge D, Fuentes E, Rose AB, Debus SJS (2010) Diets of wedge-tailed eagles (Aquila audax) and little eagles (Hieraaetus morphnoides) breeding near Canberra, Australia. J Raptor Res 44:50–61
Osborne RAL (1991) Red earth and bones: the history of cave sediment studies in New South Wales, Australia. Earth Sci Hist 10:13–28
Osborne RAL (1997) Rehabilitation of the Wellington Caves phosphate mine: implications for Cainozoic stratigraphy. Proc Linnean Soc NSW 117:175–180
Osborne RAL (2001) Karst geology of Wellington caves: a review. Helictite 37:3–12
Prideaux GJ, Roberts RG, Megirian D, Westaway KE, Hellstrom JC, Olley JM (2007) Mammalian responses to Pleistocene climate change in south-eastern Australia. Geology 35:33–36
Reed EH (2003) Taphonomy of large mammal bone deposits, Naracoorte Caves. Unpublished PhD thesis, Flinders University of South Australia, Australia
Reed EH (2006) In situ taphonomic investigation of Pleistocene large mammal bone deposits from The Ossuaries, Victoria Fossil Cave, Naracoorte, South Australia. Helictite 39:5–15
Rich P, van Tets GF (1982) Fossil birds of Australia and New Guinea: their biogeographic, phylogenetic and biostratigraphic input. In: Rich PV, Thompson EM (eds) The fossil vertebrate record of Australasia. Monash University Offset Printing Unit, Clayton, pp 235–384
Rich P, van Tets GF, McEvey AR (1982) Pleistocene records of Falco berigora from Australia and the identity of Asturaetus furcillatus De Vis (Aves: Falconidae). Mem Queensl Museum 20:687–693
Saltre F, Rodríguez-Rey M, Brook BW, Johnson CN, Turney CSM, Alroy J, Cooper A, Beeton N, Bird MI, Fordham DA, Gillespie R, Herrando-Pérez S, Jacobs Z, Miller GH, Nogués-Bravo D, Prideaux GJ, Roberts RG, Bradshaw CJA (2016) Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat Commun 7:1–7
Sanders WJ, Trapani J, Mitani JC (2003) Taphonomic aspects of crowned eagle-Hawk predation on monkeys. J Hum Evol 44:87–105
Simpson K, Day N (2010) Field guide to the birds of Australia, 8th edn. Penguin Group, Camberwell
Stegmann BC (1978) Relationships of the superorders Alectoromorphae and Charadriomorphae (Aves): a comparative study of the avian hand. Publ Nuttall Ornithol Club 17:1–119
Tedford RH, Wells RT (1990) Pleistocene deposits and fossil vertebrates from the “Dead heart of Australia.” Mem Queensl Museum 28:263–284
Treble P, Baker A, Ayliffe LK, Cohen TJ, Hellstrom JC (2017) Hydroclimate of the Last Glacial Maximum and deglaciation in southern Australia ’ s arid margin interpreted from speleothem records (23–15 ka). Clim past 13:667–687
Treinys R, Dementavičius D, Mozgeris G, Skuja S, Rumbutis S, Stončius D (2011) Coexistence of protected avian predators: does a recovering population of White-tailed Eagle threaten to exclude other predators? Eur J Wildl Res 57:1165–1174
Van Tets GF, Rich PV (1990) An evaluation of De Vis’ fossil birds. Mem Queensl Museum 28:165–168
Vieillot LJP (1816) Analyse d’une nouvelle ornithology elementaire. D’eterville, Paris
Vigors NA (1824) Sketches in ornithology; or, observations on the leading affinities of some of the more extensive groups of birds. On the groups of Falconidae. Zool J 2:308–346
Weij R, Woodhead JD, Sniderman JMK, Hellstrom JC, Reed E, Bourne S, Drysdale RN, Pollard TJ (2022) Cave opening and fossil accumulation in Naracoorte, Australia, through charcoal and pollen in dated speleothems. Commun Earth Environ 3:2. https://doi.org/10.1038/s43247-022-00538-y
Whitfield DP, Marquiss M, Reid R, Grant J, Tingay R, Evans RJ (2013) Breeding season diets of sympatric White-tailed Eagles and Golden Eagles in Scotland: no evidence for competitive effects. Bird Study 60:67–76
Worthy TH, Holdaway RN (2002) The lost world of the moa: prehistoric life of New Zealand. Indiana University Press, Bloomington
Worthy TH, Yates A (2018) A review of the smaller birds from the late Miocene Alcoota local faunas of Australia with a description of a new anatid species. Contrib Del MACN 7:221–252
Worthy TH, Mitri M, Handley WD, Lee MS, Anderson A, Sand C (2016) Osteology supports a stem-galliform affinity for the giant extinct flightless bird Sylviornis neocaledoniae (Sylviornithidae, Galloanseres). PLoS ONE. https://doi.org/10.1371/journal.pone.0150871
Wroe S, Field JH, Archer M, Mooney SD (2013) Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea). PNAS 110:8777–8781. https://doi.org/10.1073/pnas.1302698110
Zhang Z, Huang Y, James HF, Hou L (2012) Two Old World vultures from the middle Pleistocene of northeastern China and their implications for interspecific competition and biogeography of Aegypiinae. J Vertebr Palaeontol 32:117–124
Acknowledgements
We would like to thank the following people for their contributions to our research: Dee Trewartha, Andrew Stempel, Sarah Gilbert, William Cooper, and David Mansueto of the Flinders University Speleological Society Inc. for their invaluable caving experience and assistance in collecting fossil material. Ian D Lewis, the president of the Cave Exploration Group (South Australia), for giving permission for usage of the map of Mairs Cave that was produced by CEGSA in this paper. Jon and Bek Rasheed, the landowners who gave us permission to stay on their property and visit the cave. Elizabeth Scharsachs and her family, for providing accommodation in Tring. Judith White, for providing photographic images for specimens of Torgos tracheliotos, Trigonoceps occipitalis, Sarcogyps calvus and Gypaetus barbatus. We thank Phillipa Horton, Maya Penck and Mary-Anne Binnie (SAMA), Jacqueline Nguyen and Matthew McCurry (AM), Judith White (NHMUK), Chris Milensky (USNM), Mark Robbins (KU), Tim Ziegler and Karen Roberts (NMV), and Leo Joseph and Alex Drew (ANWC) for allowing access to their respective collections and specimens. Finally, we thank our reviewers, Gerald Mayr and an anonymous individual, for evaluating the manuscript and providing valuable feedback and critique.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
EKM and THW designed the study. EKM, THW and ABC were involved in the collection of more fossil material for the purpose of the study. All authors contributed to the manuscript, with EKM writing the initial draft and THW, MSYL and ABC providing feedback, revisions, and suggestions. EKM was responsible for the comparisons and descriptions of fossil material, with THW providing corrections and feedback. MSYL collated the molecular data, while EKM collected the morphological data and performed analyses. Funding for the research was provided by the Australian Postgraduate Scholarship and ARC Funding.
Corresponding author
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mather, E.K., Lee, M.S.Y., Camens, A.B. et al. A giant raptor (Aves: Accipitridae) from the Pleistocene of southern Australia. J Ornithol 164, 499–526 (2023). https://doi.org/10.1007/s10336-023-02055-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02055-x