Abstract
Climate change poses significant impact on most migratory species, altering their breeding and non-breeding distributions, migration distances, and phenology. The ability of a population to track and adjust to the changing environmental conditions is a vital prerequisite for its persistence. In this study, we describe changes in spring arrival phenology of a short-distance migrant, the Common Starling Sturnus vulgaris, in Northeastern Europe and their relation to changes in the onset of meteorological spring during the last century. Phenological data were obtained from an open access database Chronicles of Nature Calendar that held 4291 usable records of the 1st spring observation date of Starlings from 171 phenological network sites between 1922 and 2017. Air temperature data were downloaded from the European Climate Assessment and Dataset database. We found that spring arrival time of Starlings was positively correlated with the onset of spring on a local and broad scale. Spring arrival progression followed a northeast gradient at an average rate of 1.6 days per one degree of latitude (ca. 71 km day−1) and 0.4 days per one degree of longitude (152 km day−1). During the 96-year long period, Starlings on average advanced their spring arrival time by 4.7 days at a rate of 0.05 days per year. However, changes in the onset of spring occurred more rapidly with an average rate of change of 0.18 days per year over the study period. These findings imply broad-scale adjustments in migratory patterns of a short-distance migrant that result from climate change-induced shifts in spring phenology. However, adaptations in bird migration phenology are not occurring fast enough to track and compensate for the rapid increase in air temperature, thus disrupting the earlier matching between bird arrival and spring onset.
Zusammenfassung
Frühlingsphänologie verfrüht sich schneller als die Ankunftszeiten von Staren.
Der Klimawandel hat erhebliche Auswirkungen auf die meisten wandernden Arten; er verändert ihre Verbreitung in den Brut- und Überwinterungsgebieten, die zurückzulegenden Zugstrecken und die Zugphänologie. Die Fähigkeit der Populationen, die sich ändernden Umweltbedingungen zu erfassen und sich daran anzupassen, ist eine wesentliche Voraussetzung für ihren Fortbestand. In der vorliegenden Studie beschreiben wir Veränderungen in der Frühlingsankunftsphänologie des Kurzstreckenziehers Star (Sturnus vulgaris) in Nordosteuropa während des letzten Jahrhunderts und die Verbindung zu Änderungen im Beginn des meteorologischen Frühlings. Die zugrundeliegenden Daten stammen aus der frei verfügbaren Datenbank des phänologischen Netzwerks „Chronicles of Nature Calendar “ und umfassen 4291 verwertbare Erstbeobachtungen von Staren an 171 Standorten zwischen 1922 und 2017. Die dazugehörigen Lufttemperaturdaten wurden der „European Climate Assessment and Dataset “-Datenbank entnommen. Die Frühlingsankunftszeit der Stare korrelierte positiv mit dem Beginn des Frühlings auf lokaler und auf regionaler Ebene. Die Ankunftszeit verschob sich über das Untersuchungsgebiet entlang eines Nordostgradienten mit einer durchschnittlichen Rate von 1,6 Tagen pro Breitengrad (ca. 71 km pro Tag) und 0,4 Tagen pro Längengrad (152 km pro Tag). Während des 96-jährigen Untersuchungszeitraums verfrühte sich die Ankunftszeit der Stare im Durchschnitt um 4,7 Tage mit einer Rate von 0,05 Tagen pro Jahr. Gleichzeitig verfrühte sich jedoch der meteorologische Frühlingsbeginn weitaus schneller mit einer durchschnittlichen Verschiebung von 0,18 Tagen pro Jahr. Unsere Ergebnisse implizieren, dass auch beim Star als Kurzstreckenzieher eine Anpassung der Zugzeiten mit der Verschiebung der Frühlingsphänologie einhergeht, die durch den Klimawandel verursacht ist. Die Anpassungen der Zugphänologie erfolgen jedoch nicht schnell genug, um den raschen Anstieg der Lufttemperatur zu folgen und zu kompensieren, wodurch die zeitliche Abstimmung der Ankunft der Vögel mit dem Frühlingsbeginn gestört wird.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 1880 Earth’s average air temperature has risen by 0.85 °C (IPCC 2014) and the climate change and its resulting consequences are becoming an increasingly significant issue. Although species have always adapted to the changing climate, it is the currently rapid rate of climate change that is causing concern (O’Neill et al. 2017). Rapid climate change can lead to habitat destruction, species declines and extinction, or even complete collapse of ecosystems (Mantyka-Pringle et al. 2011; Bergstrom et al. 2021). If current trends continue, one in six species is at risk of extinction in the future due to climate change (Urban 2015).
Animal populations have shown a multitude of adaptive responses to the changing environmental conditions (Radchuk et al. 2019). Changes in species distribution ranges and phenological advances are among the most significant and visible effects seen in living organisms, particularly in birds (Crick 2004; Cohen et al. 2018). The most pronounced range shifts occur as species are expanding towards higher latitudes and retreating from lower latitudes (Thomas and Lennon 1999; Devictor et al. 2008; Chen et al. 2011; Virkkala and Lehikoinen 2017). Changes in spatial and temporal patterns of bird migration are evident not only locally but also on a global scale (Mason et al. 2019; Horton et al. 2020). For example, short-distance migratory birds return earlier in spring and start their migration later in autumn (Jenni and Kéry 2003; Jonzén et al. 2006), but some birds no longer migrate as far as before (Bradshaw and Holzapfel 2006; Visser et al. 2009). Consequently, birds are starting their breeding season earlier (Romano et al. 2022) and changes in breeding phenology have a direct impact on migration phenology and vice versa (Koleček et al. 2020).
Regarding phenology of spring migration, several recent large-scale studies have highlighted a general pattern of significant advances in migration timing (Usui et al. 2017; Cohen et al. 2018; Radchuk et al. 2019; Horton et al. 2020; Romano et al. 2022). These trends hold true across different taxa and continents. As a general pattern, short-distance migrants have shown greater advances in their arrival time compared to long-distance migrants, possibly due to greater phenotypic plasticity of short-distance migrants in response to temperature changes (Usui et al. 2017; Koleček et al. 2020). Failure to adapt spring arrival timing to changing environmental phenology may lead to phenological mismatch between peak food availability and demand (Jones and Cresswell 2010). Species and populations that fail to adapt their spring arrival phenology to climate change have been shown to decline at a faster pace than those that do (Møller et al. 2008; Saino et al. 2011).
Common Starling Sturnus vulgaris (hereafter, Starling) populations across Europe have overall declined by 68% between 1980 and 2015 but show contrasting population trends between increases in Central-East Europe and declines in North and West Europe (Heldbjerg et al. 2019). Across large parts of its breeding range in continental Europe, the Starling is a short-distance migrant (Feare 1984). Due to its lifestyle in the close proximity to human settlements Starlings are easy to observe and the species is well known to most people, making it an ideal model species for citizen science projects, including phenological research. Here, we use spring arrival data of Starlings obtained from a large-scale citizen science-based phenological network operating in Northeast Europe for nearly 100 years. Coupling it with meteorological data from nearby weather stations, we test if and at what pace the spring migration timing of the Starlings have changed relative to the pace of changes in spring onset timing. We hypothesize that Starlings have advanced their spring migration phenology in response to the warming temperatures, but these advances are insufficient to fully compensate for the ever earlier timing of spring onset (Radchuk et al. 2019).
Methods
Data acquisition and filtering
Starling phenology data were obtained from the open access phenological database Chronicles of Nature Calendar (Ovaskainen et al. 2020). This phenological network was started by the Russian Geographic society in 1848. It gradually evolved in the early 1900s until officially established in 1940. The database contains entries of a large number of different taxonomic groups—plants, birds, amphibians, reptiles, etc. The data cover various phenological measurements, for example, the dates of the first observation in spring and the last observation in autumn for migratory birds, but for plants—flowering and leaf fall times.
In this study, we selected phenological data of the spring arrival of Starlings—day of year of the first observation of the species in various places and years across the phenological network. The data covered a 96-year long period from 1922 to 2017 totalling 4682 observations from 199 observation points in eight countries: Latvia, Lithuania, Estonia, Finland, Poland, Belarus, Ukraine, and Russia. Two observation sites were located in the Altai Republic of Russia, near the Mongolian border—we excluded these points from further analysis as they are located outside of Europe and are spatially disjunct from other observation sites. Further, we excluded observations that have been marked as possible outliers in the database (see Ovaskainen et al. 2020 for more details on outlier identification) and pooled together observation sites that were less than 25 km apart from each other by averaging the recorded timing of Starling arrival. The latter was primarily done because the raw data contained pseudo-replicates of several observation sites (seemingly because of change in site IDs and coordinate approximation during the operation of the phenological network).
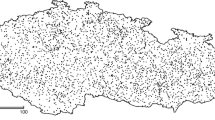
After data filtering, 4291 spring arrival time records from 171 sites between 1922 and 2017 were retained for further analyses (Fig. 1). Accordingly, the study covered a large area of northeast Europe between 47°–70° N and 21°–66° E.
Spatiotemporal distribution of the phenological data used in analyses. a Locations where the timing of Starling arrival was recorded (red triangles; size corresponds to the number of observation years) and nearby weather stations (blue dots) from which temperature data were acquired. b Yearly number of observation sites where Starling arrival was recorded across the study period
Air temperature data were obtained from the European Climate Assessment and Dataset (ECA&D, Klein Tank et al. 2002; https://www.ecad.eu/dailydata/predefinedseries.php). We downloaded daily average air temperature data from all weather stations located in the eight above-mentioned countries—a total of 1080 stations—for 1922–2017. Next, observations made between July and January were deleted from the temperature dataset, thus retaining only information on the spring period between 1 February and 30 June. Further, for each phenological observation site, we identified the nearest weather stations using the function distVincentyEllipsoid from the R-package geosphere (Hijmans 2019; Fig. 1), thus geographically linking Starling spring arrival and air temperature datasets.
Data analyses
In meteorology, the exact definition of the onset of spring is not clearly defined. The definition varies depending on country/region. In Sweden, for example, the beginning of meteorological spring is declared on the first day of seven day period with a daily average temperature above 0 °C (SMHI 2011). In this study, we used an approach that is used in Latvia and is similar to the one from Sweden—the onset of spring is defined as a day when the daily average air temperature is equal to or higher than 0 °C for five consecutive days, excluding longer periods of thaw (Maļinovskis and Grīnbergs 2020). For each meteorological station, we calculated annual timing of the onset of meteorological spring using the above-mentioned criteria. Due to gaps in daily temperature data at selected meteorological stations, we could not estimate the onset of spring in 226 cases (5.3% of all Starling arrival observations).
We used kriging, to interpolate and visualize spatial patterns in the timing of spring onset and Starling arrival using the autoKrige function from the R-package automap (Hiemstra et al. 2009). AutoKrige fits a variogram model to the given dataset and makes predictions on new locations (interpolate) based on the fitted variogram model. Interpolation was done on a 0.25-degree × 0.25-degree grid across the breeding range of the Starling (BirdLife International and Handbook of the Birds of the World 2019) between 20° and 70° E in the eight above-mentioned countries, excluding southern regions of Russia due to lack of phenological data from this area.
We used Pearson's correlation to determine the strength of the relationship between the onset of spring and the arrival time of Starlings. Before testing for the correlation, we de-trended the time series of both parameters by calculating the least squares regression and using residuals of the regression for further analyses. This was done for each observation site independently (site-specific trends) as well as on annual mean timing of both parameters across all sites with available data.
We estimated the progression of Starling spring arrival time across latitudes and longitudes using multiple regression. Furthermore, we modelled temporal changes in the spring onset and Starling arrival times between 1922 and 2017 using linear mixed effects models with arrival/spring onset time as a response variable and year as an explanatory variable. Observation sites were included as a random factor in the models to account for the uneven spatiotemporal data availability (Fig. 1). Finally, we used linear regression to test for potential changes in the synergy between spring onset and Starling arrival in northeast Europe during the 96-year period. All data analyses were done in R version 4.0.2 (R Core Team 2020).
Results
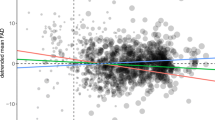
The average spring arrival date of Starlings in the entire study area from 1922 to 2017 was 89th calendar day (88.7 ± 6.5 SD) which corresponds to March 30. The average timing of spring onset was 92.9 ± 9.7 SD calendar day (April 3). After detrending the time series of Starling arrival and spring onset observations, the timing of Starling spring arrival on a local scale (i.e., sites-specific) showed a moderate correlation with the local timing of spring onset (r(4063) = 0.324, p < 0.001; Fig. 2a). On a broad scale across the entire study area, however, the annual mean arrival times showed a strong correlation with the annual mean timing of spring onset (r(94) = 0.724, p < 0.001; Fig. 2b).
Scatterplots showing the relationship between the arrival times of Starlings and the onset of meteorological spring. a Site- and year-specific timing across 171 observation sites (n = 4291), b annual mean timing from 1922 to 2017 (n = 96 years). Data have been de-trended before plotting and thus, axis values designate deviation (in number of days) from the expected linear trend (see methods). Solid lines are derived from ordinary least squares regressions where Starling arrival time was a response variable and spring onset timing was a sole predictor; dashed line designates perfect correlation where y = x
Arrival timing of Starlings showed a clear spatiotemporal trend from early arrivals in the southwest and late arrival in the north and northeast which largely matched the spatiotemporal pattern of the spring onset timing which typically lagged behind by a few days (Fig. 3). Latitude and longitude of the study sites explained 42% of the variance found in Starling arrival dates (R2 = 0.419, p < 0.001). With every degree of latitude towards north, the spring arrival time of Starlings was on average 1.6 days later (ß = 1.557 ± 0.037 SE, t = 42.163, p < 0.001) while with every degree of longitude towards east the arrival time was on average 0.4 days later (ß = 0.421 ± 0.017 SE, t = 25.013, p < 0.001, R2 = 0.394). Correspondingly, spring migration of Starlings progressed approximately at a speed of 71 km day−1 across latitudes and 152 km day−1 across longitudes (considering meridian width at 55° N = 64 km).
Mean timing of a spring arrival of the Starling and b onset of meteorological spring in NE Europe from 1922 to 2017. Coloured area represents breeding range of the Starling (BirdLife International and Handbook of the Birds of the World 2019) in nine countries—Ukraine, Moldova, Poland, Belarus, Lithuania, Latvia, Estonia, Finland, and Russia (excluding southern regions) but note that some observation sites in the northeast extend beyond the currently assumed species breeding range. Colour gradient and the dashed lines—isopipteses—display the mean timing (day of year). Dots in (a) represent observation sites where Starling arrival was recorded and in (b) nearest weather station where daily temperature data from the corresponding years was available
Between 1922 and 2017, Starling arrival time in NE Europe has on average advanced by 4.7 days at a rate of 0.05 days per year (ß = − 0.049 ± 0.01 SE, t = − 4.194, p < 0.001; Fig. 4a). At the same time, the timing of spring onset has advanced by 17.1 days at a rate of 0.18 days per year (ß = − 0.178 ± 0.01, t = − 12.96, p < 0.001). Consequently, the synergy between Starling arrival time and the timing of spring onset has also changed during this 96-year period (ß = − 0.073 ± 0.02, t = − 3.68, p < 0.001, R2 = 0.117; Fig. 4b). Throughout the twentieth century, Starlings typically arrived at their breeding sites a few days ahead of the spring onset, but this difference has diminished in recent decades.
a Changes in spring arrival timing of Starlings (red) and the onset of spring (blue) from 1922 to 2017. Dots show annual means, thin lines give SDs, and thick lines represent the overall trends with 95% confidence intervals (coloured areas) as estimated from the linear mixed-effects models. b Comparison between the annual average timing of Starling arrival and spring onset. Values above zero (red lines) indicate years when Starlings on average arrived before the start of the spring. Values below zero (blue) indicate years when the onset of spring occurred before the arrival of Starlings. Black line represents the overall trend and shaded area designates 95% confidence interval
Discussion
Our results suggest a tight link between the timing of the onset of meteorological spring and spring arrival time of Starlings. During the last 100 years phenology of both events has significantly advanced but to a different degree. While Starling have advanced their arrival phenology by ca. five days, the magnitude of spring advancement has been nearly fourfold, disrupting the historic temporal synergy between the two events.
While broad-scale phenological networks like the one used in this study provide invaluable data on phenology and its changes over time, there are several limitations to such data. First, phenological data are often recorded by citizen scientists and thus, observer bias is an indisputable part of any such network. Yet, no other sources offer similar data with such historic pedigree and geographic coverage. Furthermore, a large amount of data presented here have been gathered by professionals as part of the Chronicles of Nature research program (Ovaskainen et al. 2020). Second, in our study, the number of observation sites with available data vary across years with relatively low number of observations for early and late years (Fig. 1b), increasing the uncertainty in timing estimates for these periods and lowering the geographic representation of the data. Perhaps, some degree of advances in Starling arrival during the last 20 years and its match-mismatch with the timing of spring onset may be masked by the low number of observations in this period. Third, with varying number of observation sites (and their geographic origin) across years, derived trend estimates may originate from a systematic trend in the geographic origin of observations, e.g., continuously more southern sites in later years leading to earlier estimates of arrival timing. We checked for changes in the mean latitude and longitude of observation sites across the 96-year period and found no evidence of systematic changes over time. Additionally, we included observation sites as random variables in our models, thus accounting for the uneven spatial and temporal data distribution. Therefore, it is unlikely that the observed trend in earlier Starling arrival across the study period is simply an artefact of shifting observation site locations towards southwest or insufficient data coverage. Lastly, while change in the first arrival dates is a good indicator for phenological shifts they may not necessarily be fully representative for the arrival time of the entire population (Sparks et al. 2005) calling for more in-depth studies where feasible.
The rate of Starling arrival time advancement across the entire study period has been relatively minor compared the rate of spring advancement. This may indicate an evolutionary constraint for the maximum rate at which the Starling population breeding in northeast Europe may be able to advance its spring arrival date given their specific wintering grounds, migration routes and other ecological parameters. The start of spring migration in long-distance migratory birds have been shown to strongly correlate with the timing of breeding site arrival (Briedis et al. 2019) and thus, the triggers for initiating spring migration may ultimately limit the potential for adjusting to climate change-induced shifts in environmental phenology in long-distance migrants. In short-distance migrants, on the other hand, the timing of spring migration is generally thought to be relatively flexible via individual plasticity in accordance with the annual weather patterns (Gordo 2007) and short-distance migrants have been shown to be more responsive to changes in spring temperatures at the breeding sites compared to long-distance migrants (Kluen et al. 2017). If so, short-distance migrants should be able to track phenological changes with relative ease, contradicting our findings. In Starlings, however, photoperiod has been shown to be a defining factor for controlling the timing of the annual cycle events (Gwinner 1996)—a mechanism typically found in long-distance migrants. In this instance, it would require a microevolutionary response and an adaptive selection for earlier arrival over generations to compensate for the advancing spring phenology (Gienapp et al. 2007; Gill et al. 2013)—a process that may limit the maximum rate of advancement a species can exhibit.
If temporal adjustments for the start of spring migration is constrained by intrinsic or extrinsic cues, birds still have the possibility for increasing their migration speed to compensate for the late departure from the wintering sites. However, comparative analyses that include 49 tracking studies of 46 bird species showed limited capacity of migratory birds to compensate for climate change-induced phenological advancement by increasing their migration speed (Schmaljohann and Both 2017). Furthermore, geolocator tracking of Starlings breeding in the Baltics and wintering in the British Isles has shown that spring migration are on average only two weeks long with an average migration speed of ca. 200 km day−1 (Vīgants et al. 2023) which is at the higher end of values found across passerine migrants (Schmaljohann 2018; Briedis et al. 2020). Thus, further increase in migration speed to advance arrival timing seems questionable. The speed of migration progression found in our study (ca. 71 km day−1 across latitude 152 km day−1 across longitude) is noticeably smaller compared to the numbers reported from geolocator tracking (Vīgants et al. 2023), however, they should not be directly compared as tracking-based estimates correspond to single individuals across their full migration cycle, while estimates in our study are not individual-based and represent only the final leg of migration. A large-scale study in Europe analysing different migration characteristics of 55 populations of 23 bird species found that migrants cross one degree of latitude in 1.5 days on average (Briedis et al. 2020), which is in line with our findings.
As a general pattern, short-distance migrants have been shown to better track phenological changes compared to long-distance migrants (Both et al. 2010; Kullberg et al. 2015). However, similar studies in Starlings found no significant advancement of spring arrival times on single sites in Croatia (Dolenec 2015), Poland (Biaduń et al. 2011), and Finland (Lappalainen et al. 2008). These studies, however, cover partly different and much shorter time periods (34–40 years) than our analyses presented here (96 years). Our analyses on site-specific trends yielded large variation in the observed changes in spring arrival timing of Starlings with some sites showing advances while others indicated no changes or even delayed arrival over the observation period (Supplementary materials Fig. S1). Site-specific conditions, observer-bias as well as climatic anomalies during the observation period are among the most likely causes behind these contrasting results on single geographic locations. However, our results on large-scale arrival time advancement in Northeastern Europe are unambiguous and in line with the advancement in predicted egg laying date of Starlings in this region (Both and te Marvelde 2007). Empirical data of nest records from Finland support this showing an advancement in the beginning of the breeding season by 0.16–0.2 days per year since the 1960s (Kluen et al. 2017; Hällfors et al. 2020).
Early arrival can also increase the risk for extreme weather exposure that may result in higher mortality rates for pioneer individuals (Brown and Brown 1998; Briedis et al. 2017), thus eliminating individuals with predisposition for early arrival from the population and slowing down the overall advancement of the population. This may be particularly relevant for migrant species arriving early in spring, like the Starling. Alternatively, there may be no need to advance the arrival time at a greater rate as that would increase the time between arrival and start of egg laying. If late arriving individuals can attain similar breeding success, early arrival may expose individuals to greater risk of extreme weather conditions without direct benefit for their fitness. This, however, seems to be unlikely since a recent tracking study from Latvia showed that the time interval between spring arrival and onset of egg laying is relatively long—approx. one month on average (Vīgants et al. 2023)—implying that early arrival may bear importance.
Progressing climate change will pose increased challenges for many animal species. Changes in phenology of bird migration is only one way in which migratory birds seek to adapt to the shifting environmental phenology. How successful the adaptation is, depends on the species ecology, its phenotypic plasticity, mechanisms underlying the regulation of migration, as well as the pace of climate change. Species that failed to show a phenological response to the changing climate are declining more rapidly than the ones who did (Møller et al. 2008; Saino et al. 2011). Thus, the fast rate of spring phenology advancement and the incomplete compensation to it by the Starling, may pose serious threats to the species that already shows negative population trends in the northern part of its distribution range (Heldbjerg et al. 2019), possibly due to phenological mismatch.
Data availability
The Datasets used in this study are freely available from Ovaskainen et al. (2020) and European Climate Assessment and Dataset (ECA&D, Klein Tank et al. 2002; https://www.ecad.eu/dailydata/predefinedseries.php) as well as from the authors upon request.
References
Bergstrom DM, Wienecke BC, van den Hoff J et al (2021) Combating ecosystem collapse from the tropics to the Antarctic. Glob Chang Biol 27:1692–1703. https://doi.org/10.1111/gcb.15539
Biaduń W, Kitowski I, Filipiuk E (2011) Study on the first arrival date of spring avian migrants to eastern Poland. Polish J Environ Stud 20:843–849
BirdLife International and Handbook of the Birds of the World (2019) Bird species distribution maps of the world. Version 2019.1. Available at http://datazone.birdlife.org/species/requestdis
Both C, te Marvelde L (2007) Climate change and timing of avian breeding and migration throughout Europe. Clim Res 35:93–105. https://doi.org/10.3354/cr00716
Both C, Van Turnhout CAM, Bijlsma RG et al (2010) Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc R Soc B Biol Sci 277:1259–1266. https://doi.org/10.1098/rspb.2009.1525
Bradshaw WE, Holzapfel CM (2006) Evolutionary response to rapid climate change. Science 312:1477–1478. https://doi.org/10.1126/science.1127000
Briedis M, Hahn S, Adamík P (2017) Cold spell en route delays spring arrival and decreases apparent survival in a long-distance migratory songbird. BMC Ecol 17:11. https://doi.org/10.1186/s12898-017-0121-4
Briedis M, Bauer S, Adamík P et al (2019) A full annual perspective on sex-biased migration timing in long-distance migratory birds. Proc R Soc B Biol Sci 286:20182821. https://doi.org/10.1098/rspb.2018.2821
Briedis M, Bauer S, Adamík P et al (2020) Broad-scale patterns of the Afro-Palaearctic landbird migration. Glob Ecol Biogeogr 29:722–735. https://doi.org/10.1111/geb.13063
Brown CR, Brown MB (1998) Intense natural selection on body size and wing and tailasymmetry in Cliff Swallows during severe weather. Evolution 52:1461–1475
Chen IC, Hill JK, Ohlemüller R et al (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026. https://doi.org/10.1126/science.1206432
Cohen JM, Lajeunesse MJ, Rohr JR (2018) A global synthesis of animal phenological responses to climate change. Nat Clim Chang 8:224–228. https://doi.org/10.1038/s41558-018-0067-3
Crick HQP (2004) Impact of climate change on birds. Ibis 146:48–56
Devictor V, Julliard R, Couvet D, Jiguet F (2008) Birds are tracking climate warming, but not fast enough. Proc R Soc B Biol Sci 275:2743–2748. https://doi.org/10.1098/rspb.2008.0878
Dolenec Z (2015) Relationship between spring migration, temperature and year in the Common Starling. Larus 50:29–36
Feare C (1984) The Starling. Oxford University Press
Hijmans RJ (2019) Geosphere: Spherical Trigonometry R package version 1.3–11. http://cran.r-project.org/web/packages/geosphere
Gienapp P, Leimu R, Merilä J (2007) Responses to climate change in avian migration time microevolution versus phenotypic plasticity. Clim Res 35:25–35. https://doi.org/10.3354/cr00712
Gill JA, Alves JA, Sutherland WJ et al (2013) Why is timing of bird migration advancing when individuals are not? Proc R Soc B Biol Sci 281:20132161–20132161. https://doi.org/10.1098/rspb.2013.2161
Gordo O (2007) Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim Res 35:37–58. https://doi.org/10.3354/cr00713
Gwinner E (1996) Circannual clocks in avian reproduction and migration. Ibis 138:47–63. https://doi.org/10.1111/j.1474-919X.1996.tb04767.x
Hällfors MH, Antaõ LH, Itter M, Lehikoinen A, Lindholm T, Roslin T, Saastamoinen M (2020) Shifts in timing and duration of breeding for 73 boreal bird species over four decades. Proc Natl Acad Sci 117(31):18557–18565
Heldbjerg H, Fox AD, Lehikoinen A et al (2019) Contrasting population trends of common starlings (Sturnus vulgaris) across Europe. Ornis Fenn 96:153–168
Hiemstra PH, Pebesma EJ, Twenhöfel CJW, Heuvelink GBM (2009) Real-time automatic interpolation of ambient gamma dose rates from the Dutch radioactivity monitoring network. Comput Geosci 35:1711–1721. https://doi.org/10.1016/j.cageo.2008.10.011
Horton KG, La Sorte FA, Sheldon D et al (2020) Phenology of nocturnal avian migration has shifted at the continental scale. Nat Clim Chang 10:63–68
IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. Geneva, Switzerland
Jenni L, Kéry M (2003) Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proceeding R Soc B Biol Sci 270:1467–1471. https://doi.org/10.1098/rspb.2003.2394
Jones T, Cresswell W (2010) The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? J Anim Ecol 79:98–108. https://doi.org/10.1111/j.1365-2656.2009.01610.x
Jonzén N, Lindén A, Ergon T et al (2006) Rapid advance of spring arrival dates in long-distance migratory birds. Science 312:1959–1961. https://doi.org/10.1126/science.1126119
Klein Tank AMG, Wijngaard JB, Können GP et al (2002) Daily dataset of 20th-century surface air temperature and precipitation series for the European Climate Assessment. Int J Climatol 22:1441–1453. https://doi.org/10.1002/joc.773
Kluen E, Nousiainen R, Lehikoinen A (2017) Breeding phenological response to spring weather conditions in common Finnish birds: resident species respond stronger than migratory species. J Avian Biol 48:611–619. https://doi.org/10.1111/jav.01110
Koleček J, Adamík P, Reif J (2020) Shifts in migration phenology under climate change: temperature vs. abundance effects in birds. Clim Change 159:177–194. https://doi.org/10.1007/s10584-020-02668-8
Kullberg C, Fransson T, Hedlund J et al (2015) Change in spring arrival of migratory birds under an era of climate change, Swedish data from the last 140 years. Ambio 44:69–77. https://doi.org/10.1007/s13280-014-0600-1
Lappalainen HK, Linkosalo T, Venäläinen A (2008) Long-term trends in spring phenology in a boreal forest in central Finland. Boreal Environ Res 13:303–318
Maļinovskis E, Grīnbergs DĀ (2020) Meteoroloģisko Sezonu Pārmaiņu Scenāriji Latvijai. 40
Mantyka-Pringle CS, Martin TG, Rhodes JR (2011) Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob Chang Biol 18:1239–1252. https://doi.org/10.1111/j.1365-2486.2011.02593.x
Mason LR, Green RE, Howard C, Stephens PA (2019) Population responses of bird populations to climate change on two continents vary with species ’ ecological traits but not with direction of change in climate suitability. Clim Change 157:337–355
Møller AP, Rubolini D, Lehikoinen E (2008) Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc Natl Acad Sci 105:16195–16200. https://doi.org/10.1073/pnas.0803825105
O’Neill B, Oppenheimer M, Warren R et al (2017) IPCC reasons for concern regarding climate change risks. Nat Clim Chang 7:28–37
Ovaskainen O, Meyke E, Lo C et al (2020) Chronicles of nature calendar, a long-term and large-scale multitaxon database on phenology. Sci Data 7:1–11. https://doi.org/10.1038/s41597-020-0376-z
R Core Team (2020) R: A language and environment for statistical computing
Radchuk V, Reed T, Teplitsky C et al (2019) Adaptive responses of animals to climate change are most likely insufficient. Nat Commun 10:1–14. https://doi.org/10.1038/s41467-019-10924-4
Romano A, Garamszegi LZ, Rubolini D, Ambrosini R (2022) Temporal shifts in avian phenology across the circannual cycle in a rapidly changing climate: a global meta-analysis. Ecol Monogr. https://doi.org/10.1002/ecm.1552
Saino N, Ambrosini R, Rubolini D et al (2011) Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proceedings of the Royal Society B: Biological Sciences 278:835–842. https://doi.org/10.1098/rspb.2010.1778
Schmaljohann H (2018) Proximate mechanisms affecting seasonal differences in migration speed of avian species. Sci Rep 8:4106. https://doi.org/10.1038/s41598-018-22421-7
Schmaljohann H, Both C (2017) The limits of modifying migration speed to adjust to climate change. Nat Clim Chang. https://doi.org/10.1038/nclimate3336
SMHI (2011) Vår. https://www.smhi.se/kunskapsbanken/meteorologi/var-1.1080
Sparks TH, Bairlein F, Bojarinova JG et al (2005) Examining the total arrival distribution of migratory birds. Glob Chang Biol 11:22–30. https://doi.org/10.1111/j.1365-2486.2004.00887.x
Thomas CD, Lennon JJ (1999) Birds extend their ranges northwards [4]. Nature 399:213. https://doi.org/10.1038/20335
Urban M (2015) Climate change. Accelerating extinction risk from climate change. Science 348:571–573
Usui T, Butchart SHM, Phillimore AB (2017) Temporal shifts and temperature sensitivity of avian spring migratory phenology: a phylogenetic meta-analysis. J Anim Ecol 86:250–261. https://doi.org/10.1111/1365-2656.12612
Vīgants V, Keišs O, Dinsbergs I, Jaunzemis V, Zacmane E, Priedniece A, Briedis M (2023) Migration strategies, performance and annual activity budget in a short-distance migrant, the common starling Sturnus vulgaris. J Avian Biol. https://doi.org/10.1111/jav.03080
Virkkala R, Lehikoinen A (2017) Birds on the move in the face of climate change: High species turnover in northern Europe. Ecol Evol 7:8201–8209. https://doi.org/10.1002/ece3.3328
Visser ME, Perdeck AC, van Balen JH, Both C (2009) Climate change leads to decreasing bird migration distances. Glob Chang Biol 15:1859–1865. https://doi.org/10.1111/j.1365-2486.2009.01865.x
Acknowledgements
We are thankful to all field observers and participants of the phenological network who systematically recorded their observations for almost 100 years. We thank two anonymous reviewers for their efforts and valuable comments on an earlier version of the manuscript.
Funding
This study was funded by the Latvian Council of Science (project No. lzp-2019/1-0242).
Author information
Authors and Affiliations
Contributions
MB designed the study. MB and OK acquired funding. ID compiled the data. ID and MB analysed the data and wrote the original draft which was reviewed by OK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dinsbergs, I., Keišs, O. & Briedis, M. Spring phenology is advancing at a faster rate than arrival times of Common Starling. J Ornithol 164, 367–375 (2023). https://doi.org/10.1007/s10336-022-02044-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02044-6