Abstract
The Northern German Black Grouse metapopulation has been affected since decades by a sharply decline that has ultimately led to a reduced genetic variability. Gastrointestinal parasitoses are common among Black Grouse, but it is not clear if they have a further negative impact on the development of this already threatened metapopulation. Therefore, between 2011 and 2014, the occurrence and seasonal variation of gastrointestinal parasites were assessed by coproscopical investigations of Black Grouse feces collected in the nature conservation area of the “Lüneburg Heath”. Feces were obtained also from individuals during capture and re-capture activities. In total, 1,187 fecal samples were analyzed, of which 365 were caecal and 822 were rectal feces samples. 86% of the caecal and 95% of the rectal feces samples were parasite negative. Of the positive samples, oocysts of Coccidia spp. showed the highest prevalences of 12.1% in caecal and 1.1% in rectal samples, respectively. Helminths of the species/genera Trichostrongylus tenuis, Ascaridia spp., Heterakis spp., Capillaria spp., and Syngamus trachea were also observed, but at remarkably lower prevalences. High and moderate excretion intensities were observed only for coccidian oocysts. Coccidian infections revealed a seasonal pattern, occurring mostly during autumn and winter. Nematode eggs occurred more frequently in spring and summer. Four of the seven Black Grouse that were caught and equipped with GPS transmitters, presented high excretion intensities of coccidian oocysts. Despite high/moderate oocyst excretion, no negative health impact was observed, suggesting low pathogenicity of the infecting coccidia species or subsided infections. In contrast to previous studies, this Black Grouse population showed low prevalences of a rather narrow spectrum of parasites. We did not observe any negative impact of parasite infections on this population, which could be responsible for its decline. Thus, causes of decline are to be ascribed to other proximate and ultimate factors.
Zusammenfassung
Prävalenz von Magen-Darm-Parasiten in einer Birkhuhn-Population im mitteleuropäischen Tiefland. Isolation und Fragmentierung der Birkhuhn-Metapopulation im norddeutschen Tiefland hat zu einer Verringerung der genetischen Variabilität geführt. Genetische Depression kann die individuelle Fitness und das Immunsystem beeinträchtigen, was zu einer erhöhten Anfälligkeit für Infektionen wie beispielsweise Magen-Darm-Parasitosen führen kann. Daher wurden zwischen 2012 und 2014 das Vorkommen und das saisonale Auftreten von Magen-Darm-Parasiten durch koproskopische Untersuchungen von Birkhuhnkot aus dem Naturschutzgebiet „Lüneburger Heide “ erfasst. Außerdem wurden im Rahmen eines weitergehenden Forschungsprojektes zur Habitatnutzung Birkhühner gefangen und mit GPS-Sendern versehen. Dadurch wurde das Auffinden von Kotproben unterstützt. Insgesamt wurden 1.187 Kotproben analysiert, davon 365 Blinddarm- und 822 Rektalkotproben. 86% der Blinddarm- und 95% der Rektalkotproben waren parasitennegativ. Von den positiven Proben wiesen Oozysten von Coccidia spp. die höchsten Prävalenzen von 12.1% in Blinddarm- bzw. 1.1% in Rektalproben auf. Helminthen der Arten/Gattungen Trichostrongylus tenuis, Ascaridia spp., Heterakis spp., Capillaria spp. und Syngamus trachea wurden ebenfalls nachgewiesen, jedoch mit deutlich geringeren Prävalenzen. Lediglich bei Kokzidien-Oozysten wurden hohe und mäßige Ausscheidungsintensitäten beobachtet. Kokzidieninfektionen zeigten ein saisonales Muster und traten hauptsächlich im Herbst und Winter auf. Nematodeneier waren dagegen häufiger im Frühjahr und Sommer zu finden. Von den sieben besenderten Birkhühnern wurden beim Fang Kotproben entnommen. Vier Birkhühner zeigten hohe Ausscheidungsintensitäten von Kokzidien-Oozysten. Trotz hoher/mäßiger Oozystenausscheidung wurden keine negativen Auswirkungen auf die Gesundheit beobachtet, was auf eine geringe Pathogenität der infizierenden Kokzidienspezies oder abgeklungene Infektionen hindeutet. Im Gegensatz zu früheren Studien zeigte diese Birkhuhnpopulation geringe Prävalenzen eines eher schmalen Parasitenspektrums. Wir haben keine nachteiligen Auswirkungen von Parasiteninfektionen auf diese Population beobachtet, die für ihren Rückgang verantwortlich sein könnten. Für den Rückgang dieser Birkhuhnpopulation sind somit andere mittelbare oder unmittelbare Faktoren verantwortlich.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
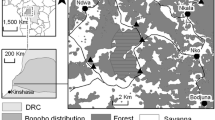
In Central Europe, Black Grouse (Tetrao tetrix) populations have been affected by sharp declines for decades as in the case of Germany as well as of the Netherlands, Belgium, Poland, and Czech Republic (Ten Den and Niewold 2000; Loneux et al. 2003; Jankovska et al. 2012; Gedeon et al. 2014; Ciach 2015). Many populations already went extinct (e.g., in Germany: Muskauer Heide, Diepholzer Moorniederung, Großes Moor near Gifhorn) (Wübbenhorst and Prüter 2007; Brozio and Schröder 2018). In Germany, the total size of Black Grouse populations is estimated at about 1,200 individuals, its largest part situated in the Bavarian Alps (Storch 2008; Gedeon et al. 2014). Outside of the Alps, the most abundant autochthonous metapopulation of Black Grouse in Germany, the Netherlands and Belgium is located in the “Lüneburg Heath”, a geographical region in the German federal state of Lower Saxony (Strauß et al. 2019; Fig. 1). To this region belongs the Lüneburg Heath nature reserve, which is one of the protected areas that have been designated as part of the “Strategy for Species and Biotope Conservation in Lower Saxony”, to preserve the last autochthonous population. In this area, habitat improvement, predator control, visitor management as well as population monitoring have been implemented (NSG-VO 1993; NLWKN 2011; Strauß et al. 2018). Nevertheless, the “Lüneburg Heath” metapopulation decreased from up to 9000 individuals in 1959, to 261 individuals in 2011, and to 130 individuals in 2019 (K. Sandkühler and NLWKN personal communication 2019). Continuous habitat loss due to peat bog drainage and mining, along with agricultural developments are some of the causes of the rapid decline (Wübbenhorst and Prüter 2007; Wormanns 2008; Ludwig et al. 2009). Moreover, isolation and fragmentation of the Black Grouse metapopulation in the “Lüneburg Heath” worsen the situation leading to a reduction in genetic variability (Segelbacher et al. 2014), and it is known that isolated and small populations are highly susceptible to further decline due to the effects of genetic drift and inbreeding (Höglund 2009). From one side, inbreeding depression has detrimental effects on the survival and reproduction of individuals (and ultimately the survival of the population) (Keller and Waller 2002). From the other side, to the best of our knowledge, it remains unclear if the reduction in genetic variability of the above-cited metapopulation could also limit the adaptability to changing environmental conditions, hinder the immune system, and thus may decrease individual fitness.

Available at http://datazone.birdlife.org/species/requestdis”
Occurrence of Black Grouse populations (black areas) in Germany and neighboring countries. The distribution shows the period 2000–2015 (modified from Strauß et al. 2019, on the basis of „BirdLife International and Handbook of the Birds of the World (2018) Bird species distribution maps of the world. Version 2018.1.
With this background in mind, parasitic infections such as gastrointestinal parasitoses may sharpen the decline of this already isolated population, negatively affecting its health. In fact, gastrointestinal parasites are widely disseminated among Black Grouse (Klaus et al. 1990; Formenti et al. 2012; Isomursu et al. 2017). Such infections, or the resulting diseases, have often been regarded as limiting factors in the populations of many tetraonid species (Zbinden and Hörnig 1985; Hudson 1986; Moss et al. 1993; Hudson et al. 1998; Obeso et al. 2000; Holmstad et al. 2005; Jankovska et al. 2012).
As part of two research projects from 2011 to 2017, the habitat quality, diet, parasite prevalence, mortality and habitat use of Black Grouse in some of the areas in the geographical region “Lüneburg Heath” were analyzed to understand the causes of the population decline (Strauß et al. 2018).
In this study, we aimed to assess the occurrence and prevalence of gastrointestinal parasites by semiquantitative analysis of fecal egg/oocyst excretion in different parts of the “Lüneburg Heath” Black Grouse subpopulation as well as among some individuals that were radio-tracked, and, moreover, we aimed to investigate the seasonal variation in parasitic infections.
Considering that increased parasite prevalences could have a negative impact on the health status of Black Grouse populations and could therefore lead to the negative development observed in the past years, this research may contribute to a better understanding of the causes of endangered Black Grouse population decline and to future regional conservation concepts.
Methods
Study area and population
The nature conservation area “Lüneburg Heath “ (hereafter referred to as NCA LH) is located in Northern Germany, south of the city of Hamburg, and is situated in a wooded, morainic landscape with mostly sandy soils. As a recreation area accessible via hiking trails, it is often visited by tourists. This heathland is one of Germany’s oldest conservation areas and has been managed by the nature park foundation “Stiftung Naturschutzpark Lüneburger Heide e.V.” for more than 100 years. Over the last decades, Black Grouse have been the focus of the area’s management concept (Wormanns 2008). One of the management’s major objectives is the conservation of juniper and sandy heath habitats, as well as the maintenance of a close connection to adjacent bogs and woodlands (Prüter and Wübbenhorst 2004; Wübbenhorst and Prüter 2007). The study area primarily coincided with the NCA LH, covering a surface area of ~ 23,400 ha that are subdivided into 66% forest, 22% heathland, 6% agricultural field, 5% pastureland, and 1% trail, settlement and water areas. Closed forests, however, are unsuitable as Black Grouse habitats because of high degrees of canopy (coverage) and older successional stages (Strauß et al. 2014). Over 5213 ha of the heathlands, including renatured bogs, provide a rich food and cover supply year-round.
Furthermore, the NCA LH, and so our study area, is divided in five core areas (where Black Grouse are regularly observed, e.g. spring count, monitoring, and lek places are known): “Radenbach Heide” (RH) and “Wilseder Berg” (WB) are located in the northern part and “Wümme Heide” (WH), “Osterheide” (OH), and other areas (SG) are located in the southwestern part. The number of animals of the subpopulation in the NCA LH increased from 25 Black Grouse in 1999 to a maximum of 78 animals in 2007. During the study period 2011–2014, between 54 and 66 cocks and hens lived in the area (Strauß et al. 2018).
Sample collection
Sample collection occurred in May 2011 and between February 2012 and September 2014 with different sampling intervals as follows. In the core areas RH and WB, fecal samples were collected from both radio-tracked and non-radio-tracked Black Grouse at weekly intervals unless between July–September 2012 and March–August 2014 as sampling was opportunistically. In the remaining core areas, fecal samples were collected unregularly as well as during random observation in the entire study area. Furthermore, during the mating season, fecal samples were collected at leks too. Overall, individual allocation of these fecal samples was rarely possible.
Samples were classified as caecal, rectal or mixed feces. Classification was visually possible due to the easily distinguishable aspects of Black Grouse caecal and rectal feces. This distinction can indicate the predilection sites of e.g., Coccidia and help to differentiate between Ascaridia or Heterakis spp. infections, which produce eggs of very similar morphology. Caecal feces consist of a dark or greenish pulp. These feces are excreted in small portions at the resting place mostly in the early morning, but also several times throughout the day (Zettel 1974). Rectal feces are cylindrical and show, depending on their freshness, a white coating of uric acid. Black grouse pass a single rectal dropping approximately every 20 min. Based on the presence of uric acid and the texture of the rectal feces, an approximate age-assessment (fresh = up to a few days; medium-old = up to a few weeks old/absence of uric acid; old = more than 3–4 weeks old/absence of uric acid and altered texture) of the samples was conducted to exclude old ones. When caecal and rectal feces had been excreted in the same place and could not be separated, they were categorized as mixed feces.
Feces findings were additionally distinguished between single, bulk and resting place samples. Single or few droppings, which were most likely excreted by the same individual, were categorized as single samples. Bulk samples were defined as rectal and/or caecal feces that were found within a radius of ten meters and had been excreted by one or more individuals. Resting place samples were composed of more than ten single droppings that were usually found in the same place, where a Black Grouse had stayed for several hours.
After collection, samples were stored at refrigerated or room temperature and examined within two days, otherwise they were stored at –18 °C for later examination.
Sample analysis
Examination of fecal samples was performed with the combined sedimentation-flotation method using zinc sulfate as a flotation medium (ZnSO4, specific gravity 1.3) according to Becker et al. (2016). About 5 g of each caecal or rectal feces sample (resting place and bulk samples) was processed in each analysis. Larger samples were thoroughly mixed prior to analysis. In some cases, smaller amounts were available, e.g., if it was a single sample. Identification of eggs and oocysts was performed microscopically at × 100 or × 400 magnification. Excretion intensity was semi-quantitatively scored as follows:
-
(–) negative (no eggs or oocysts)
-
( +) low excretion (1–5 eggs or oocysts)
-
(+ +) moderate excretion (6–10 eggs or oocysts)
-
(+ + +) high excretion (11–20 eggs or oocysts)
-
(+ + + +) massive excretion (> 20 eggs or oocysts)
Black grouse radio tracking
In the two consecutive springs of 2011 and 2012, for the purpose of this study and for related projects as above mentioned, five cocks and two hens were caught in the northeastern study area in the NCA LH using stationary live traps. First, an adspectory clinical examination was performed to ensure that captured animals were in good body and health condition. Examination included checking for normal feather development, pectoral muscle and fat coverage at the sternum, skin condition, potential ectoparasite infestations, wounds and traumas, among other things. All birds were then equipped with backpack GPS-tags, with integrated VHF-modules and accelerometers, weighing 28 or 38 g (Gruenwald, Germany). Two of them, a cock (ID 1202) and a hen (ID 1206) were re-caught after 32 days and 167 days, respectively.
Fecal samples were collected after both capture and re-capture. In addition, after release and during radio tracking with regular visual control, all animals showed normal behavior and mobility and the hens started breeding a few days after they had been provided with GPS transmitters.
Individuals were located via VHF-telemetry once a week to download the logged GPS locations (Tost et al. 2020). However, due to administrative constraints related to the protection of species, animals with GPS transmitters could not be followed directly, meaning that fecal samples could not be individually allocated and just the area of sampling could be defined. Individual sample collection apart from the catch activities was possible only for the two re-captures and for the male ID 1207, as it was killed by a goshawk after 82 days and its carcass was found.
Statistical analysis
Based on the large differences in the numbers of samples and on the partially low prevalences, descriptive statistics were applied to test parts of the results. We performed chi-square goodness of fit tests for significant differences in the parasite prevalence in caecal and rectal feces, and for significant differences in parasite prevalence depending on sample’s age assessment using the statistical software package R (R Core Team 2019). Seasonality of coccidian oocysts in caecal and rectal feces was analyzed using generalized additive models (GAMs) in the R library “mgcv” (Wood 2004). We integrated month as a cyclic cubic regression spline. We also tested the occurrence of coccidia as a presence/absence (0/1) response variable, specifying the error family as “binomial”.
Results
Occurrence of parasite infections
In total, 1187 fecal samples were collected in the study area between May 2011 and September 2014 and analyzed thereafter. Of these, 365 were caecal samples and 822 were rectal samples (Table 1). Parasite eggs or oocysts were detected in 52 (14.2%) of the caceal and 41 (5.0%) of the rectal samples (Table 1). The occurrence of parasite eggs and oocysts in caecal feces was significantly higher than in rectal feces (chi-squared test, P < 0.0001). Moreover, two of the caecal and five of the rectal feces samples showed multiple infections. Because of the small number of mixed feces samples (n = 8), these were included in the caecal samples.
Coccidia spp. were found in 12.1% of the caecal and 1.1% of the rectal feces samples, respectively (Table 1). Ascaridia spp. eggs were detected in 1.4% of the caecal feces and in 1.0% of the rectal feces. Eggs of Trichostrongylus tenuis, Capillaria spp. and Syngamus trachea only occurred in single caecal or rectal feces samples, whereas Heterakis spp. were not detected in the caecal feces samples (Table 1). In April and May 2013, unknown eggs (with the shape of Contracecum sp.) or, likely, pseudo-parasites were found in the caecal and rectal feces samples (0.5% and 2.3%, respectively).
The prevalence of parasite infection differed between the five core areas (Table 2). Only the caecal feces samples (N = 324) from the core area RH, that had been scanned for samples regularly during the period between May 2011 and September 2014, showed parasite positive results (16.0% of samples), while no parasites could be detected in caecal feces in the other four core areas (Table 2). However, the number of caecal samples from these areas was comparatively low. Concerning rectal samples, parasite prevalences between 5.2% and 13.3% were detected in three of the five core areas of Black Grouse (Table 2).
Furthermore, we did not found any significant difference in parasite prevalences between the ages of rectal feces sample (P = 0.381).
Eggs and oocysts excretion intensity
The excretion intensity of all nematode eggs was low; none of the fecal samples showed a moderate or a high nematode egg number. Different excretion intensities, however, could be detected in caecal as well as in rectal feces samples regarding coccidian oocysts (Supplementary Table S1). More in detail, a low excretion intensity was found in 25 (6.8%), a moderate in nine (2.5%), and a high in 10 (2.7%) coccidian-positive caecal samples (n = 44), respectively (Table 1). Concerning the nine rectal samples with coccidian oocysts (Table 1), excretion intensities were low in three, moderate in one and high in five samples, respectively.
A moderate or high coccidian oocyst excretion in caecal feces could be detected especially during the autumn months of 2012 and 2013 and in the winter months of January and December 2013 (Fig. 2). During the other months, there were only occasional findings (Fig. 2). However, in general, there were relatively few samples from the summer months June to August (total number of caecal and rectal samples were nine and 82, respectively) (Supplementary Tables S2, S3).
Seasonality of parasite infections
The number of coccidia positive caecal feces samples reached its peak in October 2012 (n = 14; 51.9%) (Fig. 3; Supplementary Table S2). Similarly, in the successive year, coccidian infections increased in autumn. In fact, in September 2013 coccidian oocysts were detected in 30% (n = 3) of the caecal samples (n = 10), in October (n = 3) in 66.7% (n = 2) and in November (n = 11) in 54.5% (n = 6) of the samples, respectively (Fig. 3). Coccidia prevalence was significantly lower in the spring months than in the autumn months (P < 0.001) (Table 3; Fig. 3). By contrast, the occurrence of coccidia in the rectal feces was very low, with 1.1% positive samples (N = 9), but again the prevalence was significantly lower in spring than in autumn (P < 0.025) (Fig. 4).
Interestingly, helminth eggs were detected in the caecal and rectal samples mainly in spring (Fig. 5; Fig. 6). But seasonal comparison is hampered as the numbers of samples differed substantially and were sometimes quite low. Due to low prevalence of helminth infections (Table 1), statistical tests on prevalence or seasonality differences were omitted.
Parasite coinfections
Coinfections were only detected in one caecal, one mixed and five rectal feces samples (Table 4). Just 0.6% (n = 7) of the total number of collected samples (N = 1187) showed coinfections, which means 7.5% of all positive fecal samples (n = 93) (Table 1). A moderate (+ +) or high (+ + +) excretion of coccidian oocysts in the caecal feces occurred simultaneously with a low ( +) excretion of Syngamus trachea or Capillaria spp. eggs. Of the five rectal feces samples showing coinfections, three were double-, one triple- and one quadruple-infections, respectively (Table 4).
Parasite infections in radio-tracked black grouse
Data of the radio-tracked individuals are presented in Table 5. More in detail, based on the rectal or mixed feces samples, a high excretion intensity of coccidian oocysts was detected in three cocks (ID 1101, 1201, 1204) and one hen (ID 1205) after they had been caught the first time, while no helminth eggs were detected in any of the samples.
In the feces of the two re-captured Black Grouse (female ID 1206, male ID 1202), no parasites were detected, neither at first capture, nor at re-capture. The cock ID 1207 also did not show any parasites in the second sample.
Discussion
Endoparasites are thought to play a role in the development of Tetraonidae populations (Watson and Shaw 1991; Hudson et al. 1992a, 1992b; Jankovska et al. 2012), but there is no concordance within previous studies. Indeed, some demonstrate a negative relationship between parasite burden, breeding, growth rate, and survival (e.g., Hudson 1984; Hudson et al. 1998; Isomursu et al. 2017), whereas others do not confirm these patterns (Moss et al. 1990; Höglund et al. 1992; Schei et al. 2005). This, in addition to the quite old and scarce literature, evidences the necessity for more, and more current, knowledge especially for the autochthonous population of the Northern German lowland.
Occurrence of endoparasites
Coccidia and several helminth species, among others of the genera Eimeria, Capillaria, Ascaris, Syngamus, Heterakis and Trichostrongylus, have already been found in previous studies in Black Grouse (Haase 1939; Lund 1954; Boch and Forstner 1959; Bezubik 1960; Brglez et al. 1970; Enigk and Dey-Hazra 1975; Zbinden and Hörnig 1985; Formenti et al. 2013). However, the above-cited studies from Scandinavia, the UK, Slovenia, and the Alps were conducted on shot and dissected animals. Thus, prevalences and infection intensities are not directly comparable with coproscopical studies like ours. In endangered or small populations, only coproscopical examinations can be used to ascertain individual endoparasite status and to draw conclusions about the occurrence of parasites in the population (Strauß 1996; Obeso et al. 2000; Jankovska et al. 2012). Nevertheless, the absence of parasite stages in the feces does not necessarily indicate the absence of parasites, since egg or oocyst excretion can be low and therefore fall below the sensitivity of common coproscopical methods with the consequence of false negative results. Another limitation of coproscopical techniques is that the excretion of eggs does not necessarily correlate with the intensity of infection. In grouse, a correlation between egg excretion stages and the worm burden was only reported for Trichostrongylus tenuis (Moss et al. 1990).
The difficulty of comparison with other studies that used different methodologies is highlighted by considering the markedly lower prevalences of parasites in the feces of the Black Grouse population of the NCA LH in contrast to those of the studies mentioned previously. In our study, 86% of the caecal and 95% of the rectal feces samples were parasite negative. This is in contrast also with a study from the Czech Republic, in which the analysis of 170 fecal samples of Black Grouse showed a positivity of 43% with six different parasite species, of which Hymenolopsis spp., Trichostrongylus tenuis and coccidia (Eimeria spp.) occurred most frequently (Jankovska et al. 2012). However, in both sampling years, significant differences in prevalences and intensities were observed.
In our case, the most frequently observed parasites in both caecal and rectal feces samples were coccidia. Higher infection intensities were also found only for coccidia. The various coccidian species in Black Grouse have different predilection sites in the intestine and can cause different forms of coccidiosis (Klaus et al. 1990). In this study, however, further differentiation of the observed oocysts was not possible due to partial degradation of oocyst content in the samples that were stored frozen, but it is likely that they were Eimeria species.
The Ascaridia eggs in caecal and rectal feces samples showed prevalences of 1.4% and 1.0%, respectively. This is very low in comparison with a prevalence of 24.1% for A. compar observed in the Finnish forest grouse community (Isomursu et al. 2017), where, in addition, a negative relationship between A. compar prevalence and population growth rate in Black Grouse was found. Although these findings are not directly comparable also due to different habitats and occurrence of the grouse community in Finland, the low Ascarida prevalence found in our study suggests that there was not a similar relationship in the Black Grouse of the NCA LH.
The same applies to the remaining observed parasite genera (Syngamus, Capillaria, Heterakis and Trichostrongylus) that were rarely represented in our study, with prevalences below 0.6% in fecal samples and only low excretion intensity.
Furthermore, it is noteworthy to mention the unidentified structures resembling eggs of the genus Contracaecum (family Anisakidae) that were found in April and May 2013 in the caecal and rectal feces (n = 21) of sampled Black Grouse. The genus Contracaecum has so far only been detected in piscivorous birds (e.g., great cormorant, Phalacrocorax carbo), but not in Galliformes. Nevertheless, the samples were correctly allocated to Black Grouse. In the end, it was not possible to distinguish between true and pseudoparasites, as DNA isolation from the low number of eggs failed, and thus, molecular identification could not be conducted.
In conclusion, in the sampling period, the abundance of the Black Grouse population was assessed at 50–60 animals, resulting in a population density of 1.2 individuals/km2. Thus, many of the 1187 samples analyzed during the four years period are expected to have originated from the same individuals. It can therefore be assumed that grouse populations with low population densities are less affected by parasites in comparison to populations with higher densities. While this conclusion lies in contrast to those of previous studies (Obeso et al. 2000; Jankovska et al. 2012), the low population density, the relative territoriality of the animals for a specific site in the core area, and the sampling of the same individuals over an extended period of time support the validity of our findings.
Correlation between animal density and parasite rato also applies to animals that have been kept in confined spaces such as aviaries and to reintroduced animals (Fehlberg and Pohlmeyer 1991; Strauß 1996). In fact, it has been observed that Black Grouse kept in aviaries, or those which were reintroduced to the wild, usually exhibited a higher prevalence and intensity of parasitic infections as compared to wild populations (Fehlberg and Pohlmeyer 1991; Strauß 1996).
Seasonality of parasite infections
In this study, the frequency of coccidian oocysts found in caecal feces samples was significantly higher in autumn until begin of winter than it was in spring, and also the majority of the caecal samples with moderate/high oocyst excretion intensity also originated from these months. In all seasons, the rectal feces samples showed only low oocyst excretion, but coccidia were significantly more frequently detected in these samples in autumn than in spring. In contrast, nematode eggs occurred more frequently in the spring and summer months. Likely, in the Czech Republic, the detection of coccidia and nematodes differed between species seasonally but also across the two sampling years: merely 3% of samples contained coccidia (Eimeria spp.) during spring 2002, whereas in summer 2003, coccidia were observed in 67% of the samples (Jankovska et al. 2012).
In older studies from northern Europe, even if on shot animals, coccidia infections were detected between spring and autumn (Lund 1954; Raitis and Helminen 1969), or similarly, Ascaridia and Capillaria increased from spring to autumn, and then decreased again in the winter months (Lund 1954).
We supposed that the higher parasite prevalences observed in our study in the autumn and early winter samples are likely due to an accumulation of parasite infections during the summer months. Nevertheless, due to overall low prevalences, it can be assumed that neither in winter, nor in the reproductive season, the survival rate of the Black Grouse population in NCA LH was influenced by parasitic infections, unless young infected birds die so early that this cannot be recognized with our study design.
Parasite infection of Black Grouse caught in NCA LH
Moderate and high excretion intensities in both caecal and rectal feces samples were observed only for coccidia infections, especially during autumn and winter, and in merely 2.1% of samples overall. Higher rates in the autumn months in the NCA LH can be attributed to the presence of young animals, since they are more susceptible to coccidia infections (Klaus et al. 1990). However, this contradicts the results obtained for the captured Black Grouse. Indeed, upon first capture, three of the five captured cocks and one of the two hens were found to excrete high numbers of coccidian oocysts. The high proportion of captured Black Grouse determined to have a parasitic infection does not reflect the prevalences observed in the other fecal samples. This could be a chance occurrence due to the very low sample size of captured animals (N = 7).
Based on the relative long survival time, normal movement patterns and inconspicuousness of the animals with GPS transmitters during the monitoring, it can be assumed that coccidiosis had either subsided in those initially infected animals and was not the cause of death, or that the coccidia species were not highly pathogenic, or that these animals had reached sufficient immunity. Similarly, in one reintroduction project involving radio-tracked Black Grouse, a decrease of coccidiosis was observed (Strauß 1996). The infection intensity was highest after release into the wild and declined significantly with the persistence of the Black Grouse in the area, suggesting the development of a protective immunity.
In this study, we did not observe fecal parasite stages in the re-captured Black Grouse individuals. We supposed that neither the capture event, nor the radio tracking had adverse effects on the health of the animals or led to increased susceptibility to parasite infections, due to stress related to the capture itself, for example.
Parasite coinfections
In our study area, coinfections were found in only 0.6% (n = 7) of the feces. In general, a coinfection of several helminth species in one individual is infrequent (Páv and Zajícek 1973). Older literature reported the simultaneous presence of Heterakis and Ascaridia spp. as rare (Klaus et al. 1990), whereas a correlation between the simultaneous presence of Ascaris compar and Ao. caudinflata (syn. C. caudinflata) has been more recently reported (Formenti et al. 2012).
In the NCA LH, Heterakis spp. were not observed in coinfection events. In contrast, Ascaridia was detected simultaneously with coccidia and Syngamus trachea. In this study, however, due to the low number of samples with parasite coinfections, it was not possible to make any further assumptions.
Conclusion
The examined Black Grouse population of NCA LH showed generally low prevalences of a rather narrow parasite spectrum, in comparison to other areas of central Europe. In the analyzed period, no impact of parasite infections on the health condition of the population could be observed, so that parasite infections investigated here play only a minor role in the population decline.
The long-term isolation and fragmentation of, as well as the overall low number of individuals within the Black Grouse subpopulation of the NCA LH led to a reduction in its genetic diversity (Segelbacher et al. 2014). Nevertheless, these population effects appear to have had no impact on individuals’ immune response, susceptibility to severe parasitoses, parasite prevalence or health status in general. Thus, our findings do not support the hypothesis that parasite infections were responsible for the renewed population decline over the period of 2011–2014. Further long-term sampling of the Black Grouse population is required to record changes in the occurrence and prevalence of parasites and to provide an empirically founded basis for the management of protected Black Grouse in the region of the “Lüneburg Heath”.
References
Becker AC, Kraemer A, Epe C, Strube C (2016) Sensitivity and efficiency of selected coproscopical methods – sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol Res 115:2581–2587. https://doi.org/10.1007/s00436-016-5003-8
Bezubik B (1960) Helminth parasites of black-grouse (Lyrurus tetrix L.) and capercaillie (Tetrao urogallos L.) Acta Parasitologica Polonica 8:37–46.
Boch J, Forstner MJ (1959) Untersuchungen über dem Wurmbefall des Auerund Birkwildes. Berl Munch Tierarztl Wochenschr 72:220–223
Brglez J, Rakovec R, Hribar H (1970) Die Parasiten des Birkhuhns (Lyrurus tetrix L.) aus einigen Jagdrevieren Sloweniens (Jugoslawien). Z Jagdwiss 16:32–35. https://doi.org/10.1007/BF01917308
Das Birkhuhn im Landschaftswandel der Muskauer Heide – Ein Rückblick auf 40 Jahre ehrenamtliche Beobachtungen. In Das Birkhuhn im Landschaftswandel der Muskauer Heide unter Berücksichtigung weiterer Vogelarten der Sandheiden (eds) Eigenverlag der Naturforschenden Gesellschaft der Oberlausitz e V, Görlitz, pp 7-36.
Ciach M (2015) Rapid decline of an isolated population of the black grouse Tetrao tetrix: the crisis at the southern limit of the range. Eur J Wildl Res 61:623–627. https://doi.org/10.1007/s10344-015-0923-7
Enigk K, Dey-Hazra A (1975) Zur Behandlung des Wurmbefalles bei Fasan, Rebhuhn und Birkhuhn. Wild Huhn 78:236–239
Fehlberg U, Pohlmeyer K (1991) Erkrankung des Birkhuhnes (Tetrao tetrix L.) in Volierenhaltung. Wiener Tierärztliche Monatsschrift 78:387–390
Formenti N, Viganò R, Ferrari N, Cerutti MC, Lanfranchi P (2012) Helminth community of an alpine rock partridge (Alectoris græca) population in demographic crash. in Proc. 61. Wildlife Disease Association (WDA) & European Wildlife Disease Association (EWDA) Conference, Lyon. (eds) Wildlife Disease Association
Formenti N, Viganó R, Rotelli L, Ferrari N, Cerutti M, Lanfranchi P (2013) Effect of suboptimal environment and host age on helminth community of black grouse (Tetrao tetrix) European. J Wildl Res 59:351–358. https://doi.org/10.1007/s10344-012-0681-8
Gedeon K, Grüneberg C, Mitschke A, Sudfeldt C, Eickhorst W, Fischer S, Flade M, Frick S, Geiersberger I, Koop B, Kramer M et al. (2014) Atlas Deutscher Brutvogelarten - Atlas of German Breeding Birds. (eds) Stiftung Vogelmonitoring Deutschland and Dachverband Deutscher Avifaunisten, Münster
Haase A (1939) Untersuchungen über die bei deutschen Wildhühnern vorkommenden Eimeria-Arten. In: Archiv für Protistenkunde. (eds) Fischer.
Höglund J (2009) Genetic studies of black grouse with special reference to conservation biology: a review. Folia Zool 58:135–149
Höglund J, Alatalo RV, Lundberg A (1992) The effects of parasites on male ornaments and female choice in the lek-breeding black grouse (Tetrao tetrix). Behav Ecol Sociobiol 30:71–76
Holmstad PR, Hudson PJ, Vandvik V, Skorping A (2005) Can parasites synchronise the population fluctuations of sympatric tetraonids? - examining some minimum conditions. Oikos 109:429–434. https://doi.org/10.1111/j.0030-1299.2005.13702.x
Hudson PJ (1984) The effect of parasitic infections on the population fluctuations of Red Grouse in the north of England. in Proc. 3rd Int. Grouse Symp. 1981. World Pheasant Association, Edinburgh. pp 99–105
Hudson PJ (1986) The Effect of a parasitic nematode on the breeding production of red grouse. J Anim Ecol 55:85–92. https://doi.org/10.2307/4694
Hudson PJ, Newborn D, Dobson AP (1992a) Regulation and stability of a free-living host-parasite system: Trichostrongylus tenuis in red grouse. I. monitoring and parasite reduction experiments. J Anim Ecol 61:477–486. https://doi.org/10.2307/5338
Hudson PJ, Dobson AP, Newborn D (1992b) Do parasites make prey vulnerable to predation? red grouse and parasites. J Anim Ecol 61:681–692. https://doi.org/10.2307/5623
Hudson PJ, Dobson AP, Newborn D (1998) Prevention of population cycles by parasite removal. Science 282:2256–2258. https://doi.org/10.1126/science.282.5397.2256
Isomursu M, Helle P, Rätti O (2017) intestinal parasites as potential factors in the dynamics of a fluctuating forest grouse community. Ann Zool Fenn 54:301–313. https://doi.org/10.5735/086.054.0503
Jankovska I, Bejcek V, Langrova I, Válek P, Vadlejch J, Čadková Z (2012) Black grouse in Czech Republic and its parasites. Helminthologia 49:78–81. https://doi.org/10.2478/s11687-012-0016-z
Keller L, Waller D (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241. https://doi.org/10.1016/S0169-5347(02)02489-8
Klaus S, Bergmann H-H, Marti C, Müller F, Vitovic OA, Wiesner J (1990) Die Birkhühner (Tetrao tetrix und T. mlokosiewczi). In: Ziemsen A (eds) Wittenberg Lutherstadt. Verlag
Loneux M, Lindsey JK, Vandiepenbeeck M, Charlet O, Keulen C, Poncin P, Ruwet J-C (2003) Climatic influence on Black Grouse Population dynamic in belgian hautes-fagnes nature reserve: an update. Sylvia 39:53–57
Ludwig T, Storch I, Gärtner S (2009) Large-scale land use change may explain bird species declines in semi-natural areas: the case of Black Grouse population collapse in Lower Saxony, Germany. J Ornithol 150:871–882. https://doi.org/10.1007/s10336-009-0410-6
Lund HMK (1954) Nematodes, Cestodes and Coccidia found in 136 black grouse (Lyrurus tetrix) in Norway. Statens Viltunderøkelser, Oslo
Moss R, Trenholm IB, Watson A, Parr R (1990) Parasitism, predation and survival of hen red grouse Lagopus lagopus scoticus in spring. J Anim Ecol 59:631–642. https://doi.org/10.2307/4885
Moss R, Watson A, Trenholm IB, Parr R (1993) Caecal threadworms Trichostrongylus tenuis in red grouse Lagopus lagopus scoticus: effects of weather and host density upon estimated worm burdens. Parasitology 107:199–209. https://doi.org/10.1017/s0031182000067317
NLWKN (2011) Vollzugshinweise zum Schutz von Brutvogelarten in Niedersachsen - Wertbestimmende Brutvogelarten der Vogelschutzgebiete mit höchster Priorität für Erhaltungs- und Entwicklungsmaßnahmen - Birkhuhn (Tetrao tetrix). In: Niedersächsische Strategie zum Arten- und Biotopschutz. (eds) Niedersächsischer Landesbetrieb für Wasserwirtschaft, Küsten- und Naturschutz (NLWKN), Hannover, pp 1–8
NLWKN personal communication (2019)
NSG-VO (1993) Verordnung der Bezirksregierung Lüneburg über das Naturschutzgebiet „Lüneburger Heide“ in den Landkreisen Harburg und Soltau-Fallingbostel, vom 17. Juni 1993. Page 10, Lüneburg.
Obeso JR, Rodríguez LD, Álvarez I, Eloy N, Del Campo JC (2000) Intestinal parasites in the Cantabrian capercaillie Tetrao urogallus cantabricus: a coprological study. Ardeola 47:191–195
Páv J, Zajícek D (1973) Die Parasitenfauna der Rauhfußhühner in der CCSR. Folia Venat 3:171–188
Prüter J, Wübbenhorst J (2004) Zur Situation des Birkhuhns (Tetrao tetrix) im Naturschutzgebiet Lüneburger Heide. Jahrb Des Naturwissen Schaftlichen Ver Für Das Fürstentum Lüneburg 43:73–82
R Core Team (2019) R: A language and environment for statistical computing. in R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Raitis T, Helminen M (1969) Ascaridia compar: the most frequent intestinal parasite of gallinaceous birds in Finland. Suomen Riista 21:27–39
Schei E, Holmstad PR, Skorping A (2005) Seasonal infection patterns in Willow Grouse (Lagopus lagopus L.) do not support the presence of parasite-induced winter losses. Ornis Fennica 82:137–146
Segelbacher G, Strand T, Quintela M, Axelsson T, Jansman HH, Koelewijn H-P, Höglund J (2014) Analyses of historical and current populations of black grouse in Central Europe reveal strong effects of genetic drift and loss of genetic diversity. Conserv Genet 15:1183–1195. https://doi.org/10.1007/s10592-014-0610-3
Storch I (2008) Raufußhuhn-Schutz in Mitteleuropa - ein Überblick. Zur Situation des Birkhuhns in Deutschland. A.-T.-A. f. N. (NNA).
Strauß E (1996) Untersuchungen zu möglichen Rückgangsursachen des Birkwildes (Tetrao tetrix L.) in Oberschwaben, PhD Thesis, Universität Tübingen, Gehrden.
Strauß E, Hindersin J, Siebert U (2014) Birkwild Lüneburger Heide - Habitatnutzung, Reproduktion und Verlustursachen der autochthonen Birkhuhnpopulation im NSG Lüneburger Heide 2011–2013. In: Wild und Jagd – Landesjagdbericht 2013/14. (eds) Niedersächsisches Ministerium für Ernährung, Landwirtschaft, Verbraucherschutz und Landesentwicklung, Hannover.
Strauß E, Grüntjens T, Meutzner C (2019) Faszination Birkhuhn - Die letzten Ritter der Heide. Initia Medien und Verlag, Uelzen
Strauß E, Tost D, Ratsch C, Kulow J, Stolter C, Wormanns S, Siebert U (2018) Bestandsentwicklung und Nahrungsökologie des Birkhuhns Tetrao tetrix in Niedersachsen. Der Ornithol Beob 115:261–280
Ten Den P, Niewold FJJ (2000) The Black Grouse in Netherlands Monitoring the last (?) surviving population. Cah d’Ethol 20:299–310
Tost D, Strauss E, Jung K, Siebert U (2020) Impact of tourism on habitat use of black grouse (Tetrao tetrix) in an isolated population in northern Germany. PLoS ONE 15:e0238660. https://doi.org/10.1371/journal.pone.0238660
Watson A, Shaw JL (1991) Parasites and Scottish ptarmigan numbers. Oecologia 88:359–361. https://doi.org/10.1007/BF00317578
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686. https://doi.org/10.1198/016214504000000980
Wormanns S (2008) Projekt zum Schutz des Birkhuhns im Naturschutzgebiet Lüneburger Heide. A.-T.-A. f. N. (NNA).
Wübbenhorst J, Prüter J (2007) Grundlagen für ein Artenhilfsprogramm Birkhuhn in Niedersachsen. Naturschutz Landschaftspfl. Niedersachs. H. 42. (eds) Niedersächsischer Landesbestrieb für Wasserwirtschaft Küsten- und Naturschutz (NLWKN), Hannover
Zbinden N, Hörnig B (1985) Zum Endoparasitenbefall von Birkhahn Tetrao tetrix, Alpenschneehuhn Lagopus mutus und Steinhuhn Alectoris graeca im Tessin. Der Ornithol Beob 82:117–120
Zettel J (1974) Nahrungsökologische Untersuchungen am Birkhuhn Tetrao tetrix in den Schweizer Alpen. Der Ornithol Beob 71:186–246
Acknowledgements
We wish to thank the association Verein Naturschutzpark e.V. and the foundation Stiftung Naturschutzpark Lueneburger Heide for their energetic and logistic support of this project. We particularly thank M. Zimmermann, M. Sander, S. Wormanns and S. Weber. Furthermore, we also thank all persons who supported and realized our fieldwork, especially A. Niebuhr, D. Neubauer, J. Hindersin and A. Broll, who examined some of the fecal samples as part of a study project. Moreover, we wish to thank K. Sandkuehler of the Lower Saxony Federal Ornithological Station for contributing to annual Black Grouse counting, and M. Lynch-Miller for the language proofreading of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was supported by funds of the Lower Saxony Ministry of Food, Agriculture and Consumer Protection (Nr. 406–04032/1 – 1463(E) 2011); backpack GPS-tags of the radio tracked animals were funded by Niedersächsische Bingo Umweltstiftung (U118/11G).
Author information
Authors and Affiliations
Contributions
ES and US conceived the idea and designed the study and experiment. ES and CS administrated the project and performed the experiments. ES designed the methods, TL analyzed the dat. ES, CM and CS wrote or substantially edited the paper.
Corresponding author
Ethics declarations
Data availability and materials
Raw data are available in supplemental files.
Ethical approval
Animal experiments were permitted by the ethics commission of the Lower Saxony State Office for Consumer Protection and Food Safety (LAVES, Dept. 33 Animal Welfare, permit number: 33.9–42502-04–11/0364). To minimize stress, handling was performed by a small, trained team in a quiet environment, while the animals’ heads were covered. Permits for entering the nature reserve were issued by the lower nature conservation authorities of the Harburg and Heidekreis districts.
Additional information
Communicated by I. Moore.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strauss, E., Maistrelli, C., Strube, C. et al. Prevalence of gastrointestinal parasites in a lowland Black Grouse population in Central Europe. J Ornithol 163, 1025–1037 (2022). https://doi.org/10.1007/s10336-022-02006-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02006-y