Abstract
The declines in wet-grassland breeding shorebird populations are considered to mainly result from changes in reproduction. While there is plenty of information on nest survival, little reliable information exists on local recruitment due to confounding effects of permanent emigration. Furthermore, few studies have been able to study the roles of pre- and post-fledging survival on local recruitment. Therefore, it is unclear whether local recruitment of young reflects conditions at the breeding sites or at non-breeding sites. We studied an isolated population of the endangered Southern Dunlin (Calidris alpina schinzii) breeding on the west coast of Sweden to examine (1) brood survival (probability of at least one chick fledging) by following broods fates and (2) local recruitment (survival from hatching to 1 year old) using capture-recapture data. We then examined how much of the annual variation in juvenile survival was explained by variation in brood survival. Brood survival was on average 0.58 (annual range 0.08–1.00) and explained 64% of variation in annual local recruitment. Still local recruitment was rather high for a shorebird (0.17, SE = 0.023), which reflects the isolated nature of the study population. Our results suggest that local recruitment seems to be mainly constrained by chick survival during the pre-fledging period. Therefore, management of breeding sites leading to increased brood survival, e.g., reducing predation on chicks, should have strong impacts on local recruitment and local population growth.
Zusammenfassung
Das Überleben vor, nicht nach dem Flüggewerden führt bei einem bedrohten Küstenzugvogel, dem Südlichen Alpenstrandläufer (Calidris alpina schinzii), zu Unterschieden in der Ortstreue der Jungtiere
Man geht davon aus, dass der Rückgang der in Feuchtwiesen brütenden Küstenvogelpopulationen in erster Linie auf Veränderungen in der Fortpflanzung zurückzuführen ist. Zwar gibt es viele Ergebnisse zum Überleben im Nest, aber aufgrund der verzerrenden Effekte durch viele Abwandernde, die dauerhaft wegbleiben, gibt es nur wenig zuverlässige Informationen über die ortstreuen Tiere. Außerdem konnten nur wenige Studien die Auswirkungen des Überlebens vor und nach dem Flüggewerden auf die Ortstreue untersuchen. Deshalb ist unklar, ob die die Ortstreue der Jungen von den Verhältnissen am Brutplatz oder von denen an anderen Orten, an denen nicht gebrütet wird, abhängt. Wir untersuchten an einer isoliert lebenden Population des Südlichen Alpenstrandläufers (Calidris alpina schinzii), der an der schwedischen Westküste brütet, 1) das Überleben der Brut (Wahrscheinlichkeit, dass wenigstens ein Tier ausfliegt), indem wir das weitere Schicksal der Brut verfolgten, und 2) die Ortstreue anhand von Wiederfangsdaten (Überlebende vom Schlüpfen bis zum Alter von einem Jahr). Wir überprüften anhand dieser Informationen, wie viel der jährlichen Unterschiede im Überleben der Jungtiere mit den Unterschieden im Überleben der Brut erklärt werden konnte. Die Überlebensrate der Brut betrug im Mittel 0,58 (die Spanne eines Jahres reichte von 0,08 bis 1,00) und konnte 64% der Unterschiede in der jährlichen Ortstreue erklären. Für einen Küstenvogel war die Ortstreue ziemlich hoch (0,17, SE = 0,023), was die isolierte Lage der von uns untersuchten Population widerspiegelt. Unsere Ergebnisse deuten darauf hin, dass die Ortstreue hauptsächlich vom Überleben der Küken in der Zeit vor dem Flüggewerden begrenzt wird. Deshalb müsste ein Management von Brutplätzen, das z.B. durch die Verringerung von Ausfällen durch Räuber zu einer erhöhten Überlebensrate der Brut führte, einen großen Einfluss auf die Ortstreue und das Wachstum der Population an dem Ort haben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful conservation of endangered populations requires detailed information on factors affecting the life history traits that determine the population growth rate. Shorebird populations breeding on wet grasslands have been declining in the last decades within Europe (e.g., Thorup 2006). The population declines have been attributed to changes in reproduction rather than adult survival (e.g., Roodbergen et al. 2012; Pakanen and Thorup 2016; Plard et al. 2020). Habitat changes and increased nest predation in particular have been suggested as the driving forces behind these declines (e.g., Rönkä et al. 2006; Roodbergen et al. 2012). Importantly, reproduction can be partitioned into nest survival, number of hatched young and local recruitment, but solid knowledge about the latter is relatively scarce. This is because local recruitment is the proportion of individuals that survive and return to their natal population, and permanent emigration caused by natal dispersal may bias estimates (Paradis et al. 1998). This constitutes an important lack in our understanding of population dynamics and potential management options in species such as shorebirds, as local recruitment, i.e., juvenile survival, can have a strong contribution to population growth rate (Saether and Bakke 2000). Furthermore, as juvenile survival is determined by pre-fledging and post-fledging conditions, it can be difficult to assess whether juvenile survival reflects conditions at the breeding sites or at non-breeding sites. Mortality is often highest early in life (e.g., Caughley 1966; Sullivan 1989; Stearns 1992). However, studying pre-fledging survival of precocial species is difficult and therefore few long-term studies have been able to study the relative roles of pre- and post-fledging survival on local recruitment in such species (Roodbergen et al. 2012).
Here, we examine chick survival in the endangered Southern dunlin (Calidris alpina schinzii), using long-term data from a breeding population on the west coast of Sweden (e.g., Blomqvist et al. 2010). Firstly, we followed broods from hatching until fledging or brood disappearance to estimate annual variation in the probability of at least one chick fledging (hereafter termed brood survival). Secondly, we used capture-recapture models to estimate local recruitment, i.e., survival from hatching to the age of 1 year. Finally, we examined how much of the annual variation in juvenile survival that is explained by variation in brood survival. While brood survival and juvenile survival can be expected to be correlated, their level of correlation will be low if juvenile survival is mainly affected by conditions at the non-breeding sites. In contrast, the correlation should be stronger if juvenile survival is mainly determined by factors operating at the breeding sites. For example, juvenile survival can be low if post-fledging survival is low despite high pre-fledging survival, whereas juvenile survival will always be low if pre-fledging survival is low. The study population is isolated from other populations with the nearest breeding population being about 300 km away from our study population in the province of Scania, SW Sweden (Blomqvist et al. 2010). Due to the isolation from other populations, and because all known breeding sites are monitored regularly, and dispersal between the sites is rare and controlled for, we were able to reliably estimate local recruitment (Pakanen et al. 2017).
Materials and methods
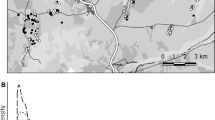
We studied Southern Dunlins on the west coast of Sweden (57°55′N, 11°47E–57°7′N, 12°14′E); here we examine chick survival at five coastal meadows that have regularly held breeding Dunlins (Fig. 1). We restrict the analyses to the data collected 1990–2005 since our data on brood survival are limited before and after this period. We started fieldwork by searching for territories in early April and continued with searching for nests or broods until late June. We usually visited the breeding sites every 2–5 days, with more frequent visits to the larger breeding sites and generally longer monitoring intervals towards the end of the breeding season. We monitored the nests until they hatched or failed. Chicks were captured in or near the nest soon after hatching and ringed with a metal ring with a numerical code that was unique for each bird (in some years, chicks were also ringed with colour rings) provided by the Swedish bird ringing centre. Broods were followed periodically every 2–3 days until the chicks were observed as fledglings (around 20 days after hatching) or they were at least 14 days old. Chick mortality is usually very low after this age (Blomqvist, unpubl.). The entire brood was considered dead if the chicks disappeared before they were 14 days old and the previously tending adults showed no parental behaviours on two or more occasions.
Location of the breeding sites of Southern Dunlin on the west coast of Sweden (1 = Ödsmåls kile, 2 = Torkelstorp, 3 = Tjolöholm, 4 = Ölmevalla, 5 = Båtafjorden, 6 = Klosterfjorden, 7 = Getterön). See Pakanen et al. (2017) for further details on the sites; note that the breeding sites Klosterfjorden and Getterön were excluded from the analyses presented here due to insufficient data on brood survival
Data on local recruitment accumulated when the chicks recruited back to population to breed in later years. We caught them as breeding adults with cage traps when they were incubating eggs or brooding chicks. We ringed each adult with a unique, individual combination of colour rings that allowed us to identify them in subsequent years. More detailed field methods can be found elsewhere (Blomqvist and Johansson 1991; Pauliny et al. 2008; Blomqvist et al. 2010; Flodin and Blomqvist 2012). The final data included 129 broods and 359 chicks that were born between 1990 and 2005. The mean number of chicks per brood at hatching was 2.7. We pooled data from each site to calculate brood survival for each year as the proportion of broods that fledged at least one chick.
We examined juvenile survival with CJS models (the Cormack-Jolly-Seber model) in program MARK (White and Burnham 1999). In this approach, individual encounter histories that summarise their observations from hatching until adulthood and until their disappearance across the different breeding seasons is used to estimate survival of individuals. The CJS model estimates survival (Φ) by correcting for the probability of recapture (p) that is estimated from the data. Our starting model [Φ(ac1t/ac2c) p(ac1t/ac2c)] included age class (ac: juveniles ac1 vs. adults ac2), but we kept survival and recapture probabilities constant in time for adults (ac2c) and time-dependent for juveniles (ac1t). This model fit the data (bootstrapping goodness of fit; p = 0.18, ĉ = 1.149). We then fitted models with constant survival and recapture probabilities for juveniles, and a covariate model that constrained juvenile survival to be a linear function of the brood survival estimate of that year. We compared models using QAICc values, which consider overdispersion and is corrected for small sample size (Burnham and Anderson 2002). To examine the relationship between temporal variation in brood survival and juvenile survival, we calculated the percentage of deviance explained by the annual covariate (cov) of brood survival as:
where Dev(c) is the deviance from the constant model, Dev(cov) is deviance from the covariate model and Dev(t) is the deviance from the time-dependent model (Grosbois et al. 2008).
Results
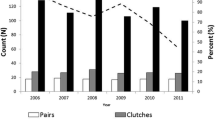
Annual brood survival varied between 0.08 and 1.00 with an annual mean of 0.58 (SE = 0.067, n = 16; Fig. 2). The best model (∆QAIC = 8.7) for juvenile survival was the covariate model showing a positive linear effect of brood survival on juvenile survival (Table 1; Fig. 3; βbrood survival = 2.290, CI 0.952–3.629 on the logit scale). Brood survival explained 64% of annual variation in juvenile survival (Fig. 3; ANODEV: F = 24.35, df = 1, p < 0.001). While annual variation in juvenile survival was not supported by the QAIC due to small sample sizes (Table 1, models A2 and A3), the annual estimates from the time-dependent model were clearly low in years of low brood survival and high in years of high brood survival (Fig. 2). Juvenile survival estimated from the constant model was 0.171 (SE = 0.023).
The relationship between brood survival and juvenile survival in a Southern Dunlin population breeding on the west coast of Sweden as estimated from model A1 (Table 1)
Discussion
We followed 129 broods of the Southern Dunlin from hatching and found that 58% of them fledged at least one chick. This means that pre-fledging survival lies between 0.19–0.58 assuming 1–3 chicks fledging from a brood. Jönsson (1991) reported a fledging survival of 0.36 for Southern Dunlins breeding in southern Sweden. While studies on pre-fledging survival from comparable small shorebirds are rare, recent studies have reported a pre-fledging survival of 0.48 for the arcticola subspecies of the dunlin (until 15 days old; Hill 2012) and 0.39–0.65 for the Piping Plover (Charadrius melanotos; until 25 days old; Hunt et al. 2018). Because those pre-fledging survival estimates measure the probability of one chick surviving, brood survival in those cases were likely higher compared to brood survival in our population. A similar study to ours estimated brood survival of 0.73 for Western Sandpipers (Calidris mauri) in Alaska (Ruthrauff and McCaffery 2005). It seems therefore that pre-fledging survival may be comparatively low in temperate grasslands. Indeed, low pre-fledging survival has been reported from larger species such as Black-tailed Godwits (Limosa limosa; 0.02–0.11; Salewski and Schütze 2017) and Lapwings (Vanellus vanellus; 0.18; Plard et al. 2020) breeding in Europe.
Average juvenile survival of Southern Dunlin in Sweden was 17%, which is slightly lower than apparent juvenile survival estimated from a Southern Dunlin population breeding at Bothnian Bay, Finland (20%, Pakanen et al. 2016) as well as in a population in southern Sweden (Skåne), where juvenile survival was estimated at 20.1% (Jönsson 1991). Notably, the latter estimate was derived using the return rate of juveniles (0.167) and assuming constant adult survival until the individuals were recruited (Jönsson 1991). The estimated survival rates for juvenile Southern Dunlins are rather high compared to other small shorebirds, in which the estimates often range between 0.05 and 0.1 because permanent emigration can occur due to available habitat elsewhere (Sandercock et al. 2005; Koivula et al. 2008; Nol et al. 2010; Pakanen et al. 2015). Since our study population shows little to no dispersal and because most of the possible breeding areas were included in the study (Fig. 1, Pakanen et al. 2017), our estimates can be considered to reliably reflect true survival. Given the apparently low pre-fledging survival, post-fledging survival needs to be rather high as the average juvenile survival, i.e., until 1 year old, was 0.17. Jönsson (1991) estimated the post-fledging survival of Southern Dunlin as 0.556.
Our estimate of juvenile survival may be too low for maintaining a stable population given other demographic rates in this population. With an adult survival rate of 0.80 for females (Blomqvist unpublished data), nest success of 0.41 (Pauliny et al. 2008), renesting probability of 0.47 (Pakanen et al. 2014) and breeding probabilities that follow those published by Pakanen et al. (2016), juvenile survival from hatching to age one should be ~ 0.26 to keep the population stable. That is 50% higher than our estimate. This level of juvenile survival was achieved in some years during the study but not in most years (Fig. 2). Thus, low juvenile survival may likely have contributed to the decline of this population (see also Plard et al. 2020).
Annual brood survival estimates explained a large portion (64%) of temporal variation in local recruitment of an endangered Southern Dunlin population. This relationship was the result of extensive variation in brood survival. In some years, brood survival was very low, allowing very little local recruitment. Based on these results, local recruitment seems to be mainly constrained by the survival during the pre-fledging period. Therefore, management actions conducted at the breeding sites that increase brood survival should have strong impacts on local recruitment. Given the rapid decline of the Baltic population of Southern Dunlin, there is a dire need for effective management that increases survival of broods. This lack of information applies to all grassland breeding species (Franks et al. 2018). Their survival can be different in different habitats; lower due to agricultural activities, low food availability or harsh weather conditions (Groen and Hemerik 2002; Lengyel 2006; Kentie et al. 2013; Ackerman et al. 2014; Machin et al. 2018). In our study population, inbreeding also affects the viability of juveniles (Blomqvist et al. 2010; see also Pauliny et al. 2008), which may be reflected in the lower survival rates especially towards the last years of the study (Fig. 2). Nevertheless, most mortality of shorebird chicks occurs through predation (Mason et al. 2018). Thus, direct predator control can be very effective (Rickenbach et al. 2011), but it requires substantial efforts that are not possible at a large scale. Therefore, identifying the habitat characteristics that lead to increased predation of chicks may lead to the most successful management options.
References
Ackerman JT, Herzog MP, Takekawa JY, Hartman CA (2014) Comparative reproductive biology of sympatric species: nest and chick survival of American avocets and black-necked stilts. J Avian Biol 45:609–623
Blomqvist D, Johansson O (1991) Distribution, reproductive success, and population trend in the dunlin Calidris alpina schinzii on the Swedish west coast. Ornis Svecica 1:39–46
Blomqvist D, Pauliny A, Larsson M, Flodin L-Å (2010) Trapped in the extinction vortex? Strong genetic effects in a declining vertebrate population. BMC Evol Biol 10:33
Burnham KP, Anderson DR (2002) Model selection and multi model inference: a practical information-theoretic approach. Springer, New York
Caughley G (1966) Mortality patterns in mammals. Ecology 47:906–918
Flodin L-Å, Blomqvist D (2012) Divorce and breeding dispersal in the dunlin Calidris alpina: support for the better option hypothesis? Behaviour 149:67–80
Franks SE, Roodbergen M, Teunissen W, Carrington Cotton A, Pearce-Higgins JW (2018) Evaluating the effectiveness of conservation measures for European grassland-breeding waders. Ecol Evol 8:10555–10568
Groen NM, Hemerik L (2002) Reproductive success and survival of Black-tailed Godwits Limosa limosa in a declining local population in The Netherlands. Ardea 90:239–248
Grosbois V, Gimenez O, Gaillard J-M, Pradel R, Barbraud C, Clobert J, Møller AP, Weimerskirch H (2008) Assessing the impact of climate variation on survival in vertebrate populations. Biol Rev 83:357–399
Hill BL (2012) Factors affecting survival of Arctic-breeding dunlin (Calidris alpina arcticola) adults and chicks. M.S. Thesis, University of Alaska Fairbanks
Hunt KL, Fraser JD, Friedrich MJ, Karpanty SM, Catlin DH (2018) Demographic response of Piping Plovers suggests that engineered habitat restoration is no match for natural riverine processes. Condor 120:149–165
Jönsson PE (1991) Reproduction and survival in a declining population of the Southern Dunlin Calidris alpina schinzii. Wader Study Group Bull 61:56–68
Kentie R, Hooijmeijer JC, Trimbos KB, Groen NM, Piersma T (2013) Intensified agricultural use of grasslands reduces growth and survival of precocial shorebird chicks. J Appl Ecol 50:243–251. https://doi.org/10.1111/1365-2664.12028
Koivula K, Pakanen V-M, Rönkä A, Belda E-J (2008) Steep past and future population decline in an arctic wader: dynamics and viability of Baltic Temminck’s stints (Calidris temminckii). J Avian Biol 39:329–340
Lengyel S (2006) Spatial differences in breeding success in the pied avocet Recurvirostra avosetta: effects of habitat on hatching success and chick survival. J Avian Biol 37:381–395. https://doi.org/10.1111/j.0908-8857.2006.03501.x
Machín P, Fernández-Elipe J, Klaassen RHG (2018) The relative importance of food abundance and weather on the growth of a sub-arctic shorebird chick. Behav Ecol Sociobiol 72:42. https://doi.org/10.1007/s00265-018-2457-y
Mason LR, Smart J, Drewitt AL (2018) Tracking day and night provides insights into the relative importance of different wader chick predators. Ibis 160:71–88. https://doi.org/10.1111/ibi.12523
Nol E, Williams S, Sandercock BK (2010) Natal philopatry and apparent survival of juvenile semipalmated plovers. Wilson J Ornithol 122:23–28
Pakanen VM, Thorup O (2016) Apparent adult survival of the critically endangered Baltic Dunlin (Calidris alpina schinzii) during a period of strong population decline. Bird Study 63:293–302
Pakanen V-M, Luukkonen A, Koivula K (2011) Nest predation and trampling as management risks in grazed coastal meadows. Biodivers Conserv 20:2057–2073
Pakanen VM, Rönkä N, Thomson R, Koivula K (2014) Informed renesting decisions: the effect of nest predation risk. Oecologia 174:1159–1167
Pakanen V-M, Lampila S, Arppe H, Valkama J (2015) Estimating sex specific apparent survival and dispersal of little ringed plovers (Charadrius dubius). Ornis Fenn 92:172–186
Pakanen V-M, Aikio S, Luukkonen A, Koivula K (2016) Grazed wet meadows are sink habitats for the southern dunlin (Calidris alpina schinzii) due to nest trampling by cattle. Ecol Evol 6:7176–7187
Pakanen VM, Koivula K, Flodin L-Å, Grissot A, Hagstedt R, Larsson M, Pauliny A, Rönkä N, Blomqvist D (2017) Between-patch natal dispersal declines with increasing natal patch size and distance to other patches in the endangered Southern Dunlin Calidris alpina schinzii. Ibis 159:611–622
Paradis E, Baillie SR, Sutherland WJ, Gregory RD (1998) Patterns of natal and breeding dispersal in birds. J Anim Ecol 67:518–536
Pauliny A, Larsson M, Blomqvist D (2008) Nest predation management: effects on reproductive success in endangered shorebirds. J Wildlife Manage 72:1579–1583
Plard F, Bruns HA, Cimiotti DV, Helmecke A, Hötker H, Jeromin H, Roodbergen M, Schekkerman H, Teunissen W, van der Jeugd H, Schaub M (2020) Low productivity and unsuitable management drive the decline of central European lapwing populations. Anim Conserv 23:286–296
Rickenbach O, Grüebler MU, Schaub M, Koller A, Naef-Daenzer B, Schifferli L (2011) Exclusion of ground predators improves Northern Lapwing Vanellus vanellus chick survival. Ibis 153:531–542
Rönkä A, Koivula K, Ojanen M, Pakanen V-M, Pohjoismäki M, Rannikko K, Rauhala P (2006) Increased nest predation in a declining and threatened Temminck’s stint (Calidris temminckii) population. Ibis 148:55–65
Roodbergen M, van der Werf B, Hötker H (2012) Revealing the contributions of reproduction and survival to the Europe-wide decline in meadow birds: review and meta-analysis. J Ornithol 153:53–74
Ruthrauff DR, McCaffery BJ (2005) Survival of western sandpiper broods on the Yukon-Kuskokwim Delta, Alaska. Condor 107:597–604
Sæther B-E, Bakke Ø (2000) Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–653
Salewski V, Schütze J (2017) Reproductive success of black-tailed Godwits in Schleswig-Holstein—a comparison of methods. Vogelwarte 55:187–198
Sandercock BK, Székely T, Kosztolányi A (2005) The effects of age and sex on the apparent survival of Kentish plovers breeding in southern Turkey. Condor 107:583–596
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Sullivan K (1989) Predation and starvation: age-specific mortality in juvenile juncos (Junco phaenotus). J Anim Ecol 58:275–286
Thorup O (2006) Breeding waders in Europe 2000. International Wader Studies 14, International Wader Study Group.
White GC, Burnham KP (1999) Program MARK: Survival estimation from populations of marked animals. Bird Study 46:120–138
Acknowledgements
We thank Lars-Åke Flodin, Mikael Larsson, Uno Unger, and Johan Wallander for excellent help during the field work. We thank Klaus-Michael Exo for valuable comments on the manuscript. The study was supported by Formas (21.5/2002-1037, 217-2005-817 and 215-2009-463, DB), funding from Oscar och Lili Lamms Minne (FO2009-0007 and FO2012-0039, AP), Carl Tryggers Stiftelse (CTS 09:294, AP), Stiftelsen Lars Hiertas Minne (AP), Stiftelsen Olle Enqvist Byggmästare (DB), as well as by the County Administration Board of Halland, Sweden. The work complies with the current laws of Sweden. Permissions for trapping and ringing in Sweden were issued by the Bird Ringing Centre (Swedish Museum of Natural History, Stockholm).
Funding
Open access funding provided by University of Oulu including Oulu University Hospital. The study was supported by Formas (21.5/2002-1037, 217-2005-817 and 215-2009-463, DB), funding from Oscar och Lili Lamms Minne (FO2009-0007 and FO2012-0039, AP), Carl Tryggers Stiftelse (CTS 09:294, AP), Stiftelsen Lars Hiertas Minne (AP), Stiftelsen Olle Enqvist Byggmästare (DB), as well as by the County Administration Board of Halland, Sweden.
Author information
Authors and Affiliations
Contributions
Data collection: DB and AP, development of question: VMP, RH and DB, data analysis: VMP, RH and DB, writing of paper: VMP, RH, AP and DB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pakanen, VM., Hagstedt, R., Pauliny, A. et al. Survival during the pre-fledging period rather than during post-fledging drives variation in local recruitment of an endangered migratory shorebird, the Southern Dunlin Calidris alpina schinzii. J Ornithol 162, 119–124 (2021). https://doi.org/10.1007/s10336-020-01814-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-020-01814-4