Abstract
Among the many richly coloured birds, hummingbirds with their brilliant colouration are outstanding. We studied the plumage of male and female Blue-throated Starfrontlet, Coeligena helianthea, which exhibits a marked sexual dichromatism. The wide diversity of coloured feathers (blue, purple, golden, green, red) makes it an attractive species to investigate the structural basis of the colouration and to study the connection between the displayed colours and the perception by conspecifics. We analysed the optical properties of the feather barbules, applying spectrophotometry, scatterometry, and electron microscopy. Using the anatomical results, the spectral data can be interpreted by optical modelling. The reflectance spectra of the feathers of male C. helianthea strikingly overlap with the spectral sensitivities of bird photoreceptors, which suggests that the feather and photoreceptor spectra are tuned.
Zusammenfassung
Sexueller Dichromatismus des Gefieders des Blaukehlmusketiers, Coeligena helianthea
Unter den vielen farbenprächtigen Vögeln stechen die Kolibris dank ihrer brillanten Färbung besonders heraus. Wir haben das Gefieder des männlichen und weiblichen Blaukehlmusketiers, Coeligena helianthea, untersucht welches einen ausgeprägten sexuellen Dichromatismus aufweist. Die große Vielfalt farbiger Federn (blau, lila, golden, grün, rot) macht diesen Vogel nicht nur zu einer attraktiven Art, um die strukturellen Grundlagen der Färbung zu untersuchen, sondern erlaubt es auch, den Zusammenhang zwischen den Federfarben und deren Wahrnehmung durch Artgenossen zu untersuchen. Wir analysierten die optischen Eigenschaften der gefärbten Bogenstrahlen mit Hilfe von Spektroskopie, Scatterometrie und Elektronenmikroskopie. Anhand der anatomischen Strukturuntersuchungen konnten wir die Reflexionsspektren durch optische Modellierung interpretieren. Die Reflexionsspektren der Federn von männlichen Blaukehlmusketieren zeigen eine spektrale Übereinstimmung mit den spektralen Empfindlichkeiten der Vogelphotorezeptoren, was darauf hindeutet, dass die Federreflexions- und die Photorezeptorspektren aufeinander abgestimmt sind.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals feature colourful patterns generated by pigmentary and/or structural colouration mechanisms, which is especially the case in birds (Hill and McGraw 2006; Kinoshita 2008; Sun et al. 2013). Several bird species engage in conspicuous displays with bright plumages created by stacks of strongly coloured feathers. The colour-producing elements can reside in different parts of the bird feather, which is a hierarchical structure made up of a main axis (the rachis), secondary axes (barbs), and tertiary axes (barbules) (Hill and McGraw 2006). In most species, the barbs and barbules are coloured by pigments. The barbs are structurally coloured when they contain quasi-ordered keratin, e.g. in Blue Jays, Cotingas, and Kingfishers (Dufresne et al. 2009; Prum et al. 2009; Stavenga et al. 2011a). The barbules are structurally coloured when they harbour regularly ordered melanosomes, organelles containing melanin. For instance, the breast feathers of the Bird of Paradise Parotia lawesii as well as the shiny feathers of the common Bronzewing, Phaps cholcoptera, contain layered stacks of solid melanin rodlets (Stavenga et al. 2011b; Xiao et al. 2014). Layers of air-filled cylindrical melanosomes colour the barbules of starling feathers (Durrer and Villiger 1970).
In hummingbirds, multilayer stacks of air-filled melanosome platelets cause the extremely iridescent colours of the feathers (Greenewalt et al. 1960a, b; Durrer 1977; Bleiweiss 1985, 1999; Osorio and Ham 2002; Giraldo et al. 2018). The periodicity of the melanosome multilayers and the properties of their components, i.e. the filling factors of melanin, keratin, and air, determine the effective refractive indices of the layers and, consequently, the reflectance spectrum of the barbules (Giraldo et al. 2018). As the melanosome stacks in hummingbird feathers closely approximate ideal multilayer reflectors, their reflectance is maximal when the detection angle equals the mirror angle, which can be manipulated during male displays (Simpson and McGraw 2018a, b). For instance, in the male Anna´s Hummingbird, the barbule planes are tilted with respect to the feather plane, so that when the male shows his colourful throat feathers while hovering in front of a female, incident sunlight is brightly and colourfully reflected to her (Stiles 1982). Because of the oblique light incidence, the hue of the feathers is shifted towards shorter wavelengths with respect to the hue of light reflected at normal light incidence (Meadows et al. 2011; Giraldo et al. 2018). Male hummingbirds display their bright iridescent feather patches (usually the crown and gorget) during aggressive, territorial, or courtship displays (Pitelka 1951; Hogan and Stoddard 2018; Simpson and McGraw 2018a, b), while duller feathers that cover the belly, upper back, and rump patches presumably are involved in crypsis (Parra 2010).
The colourful displays of animals, offering true, misleading, or false information to both receivers and exploiters, have been widely interpreted within the context of communication and signalling, because behavioural or physiological responses can shape the evolution of the sensory systems and communication strategies (Endler 1993; Endler et al. 2005). An excellent model for studying colour evolution, speciation, and biogeography along humid montane forests is the hummingbird genus Coeligena, which comprises ~ 11 species that inhabit the humid Andes from Venezuela to central Bolivia (Parra et al. 2009). Sexual selection presumably plays an important role in shaping the diversity within Coeligena species at a population and geographic level. Other mechanisms such as geographic isolation and natural selection might be essential to understand diversification events in the genus (Parra 2010). The genus is remarkable because of its colourful plumage, but little is known about the details of the mechanisms generating its structural colours. Here, we study the iridescent colouration of the Blue-throated Starfrontlet, Coeligena helianthea, which occurs in the eastern Andes of Colombia and Venezuela. The males exhibit almost the whole gamut of visible colours, including blue, green, and rosy pink. We discuss the possible relationship of these interesting characteristics with the colour perception of hummingbirds.
Materials and methods
Animals and microphotography
Feather samples from five individuals of Coeligena helianthea were obtained through loans from various museums. The feathers were photographed with an Olympus stereoscope (Olympus SZX16 stereo zoom microscope) equipped with an SDF PLAPO 1XPF objective and an Olympus SC-30 digital colour camera.
Spectrophotometry
Reflectance spectra of the feathers were recorded with an AvaSpec-2048 spectrometer using a bifurcated reflection probe (Avantes, Apeldoorn, The Netherlands). The light source was a deuterium-halogen lamp [Avantes AvaLight-D (H)-S], which illuminated approximately one square millimetre of the intact feathers. In addition, reflectance spectra of single barbules were measured with a microspectrophotometer (MSP) consisting of an AvaSpec-2048 spectrometer connected by a fibre optic to a Leitz Ortholux epi-illumination microscope, equipped with an Olympus LUCPlanFL N 20x/0.45 objective. The light source was a xenon arc lamp. The reflectance was measured from a square area of a single barbule (side length 5–10 µm). Glass UV absorption restricted the MSP measurements to wavelengths > 350 nm. All microphotographs (Fig. 1B) and reflectance spectra (Fig. 1C) were taken by tilting and rotating the feathers in a three degrees-of-freedom stage until the reflection was maximal. As a standard white diffuse reflector (WS-2, Avantes) was used as reference, the strong directional reflection of the barbules caused a severe overestimation of the reflectance, and we therefore divided all measured spectra by an estimated factor of four.
The hummingbird Coeligena helianthea. A–D Male, E–H female. A The male with sampled patches: rump (r), upper back (u), crown (c), gorget (g), and belly (b). B Microphotographs of barbs with barbules in the sampled patches; bar: 200 μm. C Reflectance spectra measured with a bifurcated probe. D Reflectance spectra measured with a microspectrophotometer. E The female with sampled patches. F Microphotographs of barbs with barbules in the sampled patches; bar: 200 μm. G Reflectance spectra measured with a bifurcated probe. H Reflectance spectra measured with a microspectrophotometer
Imaging scatterometry
In order to study the spatial reflection properties of the barbules, we applied imaging scatterometry on isolated barbs that were glued to the tip of a glass micropipette and subsequently positioned in the focal point of the ellipsoidal mirror of the scatterometer (Stavenga et al. 2009). The far-field scatterograms were recorded with an Olympus DP70 digital camera (Olympus, Tokyo, Japan).
Transmission electron microscopy
To investigate the anatomy of the barbules, we performed transmission electron microscopy. We therefore cut feather barbs with attached barbules and incubated them in 0.25 M sodium hydroxide and 0.1% Tween 20 for 30 min on a bench-top shaker. The barbs were then transferred to a 2:3 (v/v) solution of formic acid and ethanol for 2.5 h, subsequently dehydrated, by incubating in 100% ethanol twice (20 min) and 100% propylene oxide once (20 min), and infiltrated with Epon 812 in successive concentrations of 15, 50, 70 (48 h each), and 100% (24 h). We placed the barb sections into moulds, and the blocks were then cured in an oven at 20 °C for 24 h. Finally, we sliced the moulds using a diamond knife on an RMC MT-X ultramicrotome (Boeckeler Instruments, Tucson, AZ, USA) to obtain transversal sections relative to the barb. Sections were placed on 200-mesh copper grids (EMS, Fort Washington, PA, USA) with formvar support, post-stained in osmium and lead citrate, and viewed on a Phillips EM301 (Veeco FEI Inc, Hillsboro, OR, USA).
Optical modelling
We calculated the barbule reflectance spectra with a transfer matrix procedure, as before in the study of the feathers of the male Anna’s Hummingbird (Giraldo et al. 2018). We first derived from the anatomy the thicknesses of the melanosome membrane (a), the air layer (b), the cortex (c), the spacing between the melanosomes (d), the membranes within the melanosomes (e), as well as the melanosome width (w); for a diagram of the melanosome stack with the parameters, see supplementary Fig. S1. The values were implemented in the transfer matrix calculation program and then slightly varied to obtain reflectance spectra corresponding with the measurements. Gorget spectra were calculated for a = 25 nm; b = 80, 85, and 90 nm; c = 200 nm, d = 20 nm, e = 13 nm, w = 1000 nm, with N = 10 layers, and belly spectra were calculated for a = 40 nm; b = 95, 100, and 105 nm; c = 50 nm, d = 20 nm, e = 20 nm, w = 1000 nm, with N = 6 layers.
Photoreceptor excitation
We investigated the possible tuning of the feather reflectance spectra to the sensitivity spectra of the photoreceptors. From the large set of bird photoreceptors reported by Hart and Vorobyev (2005), we took the photoreceptor spectra of the well-studied Blue Tit and applied the spectral filter of the ocular lenses as in Stavenga and Wilts (2014). Furthermore, we shifted the normalized reflectance spectra of the gorget, crown, and belly along the abscissa, keeping the spectral shape constant, assuming a constant illumination spectrum, and calculated the effective absorption of the feather reflections by the three different photoreceptors as a function of the reflectance peak wavelength. We also performed the calculations for a daylight (D65) instead of a constant, wavelength-independent spectrum, but that yielded virtually identical effective absorption spectra.
Results
Colour appearance and feather reflectance
Coeligena helianthea males (Fig. 1A) display highly iridescent feather patches that range from violet to golden-red, making it a suitable model to study the structural mechanisms generating colours throughout the visible spectrum occurring among the genus. We isolated feathers of the differently coloured areas [rump (r), upper back (u), crown (c), gorget (g), and belly (b); Fig. 1A] to analyse their optical and morphological properties. Figure 1B presents microphotographs of the five studied feather areas.
To quantify the optical properties of the various feather patches, we measured the reflectance spectra using a bifurcated probe (Fig. 1C); the feathers were tilted and rotated until a reflection maximum was reached. The male gorget feather reached a remarkably high blue reflectance (~ 0.5, peak wavelength ~ 460 nm; Fig. 1Bg, C), and also the green crown had a high reflectance peak (~ 0.4, at ~ 540 nm; Fig. 1Bc, C). The reflectance spectrum of the brown upper back feather, which goes from below 0.02 at short wavelengths to about 0.05 at long wavelengths, is characteristic for a melanin-containing feather (Fig. 1Bu, C).
As the probe measurements yield the reflectance of a millimetre-sized area, we also performed measurements on single barbules with a microspectrophotometer. The reflectance spectra thus obtained were in close agreement with the probe spectra concerning the shape, but the amplitudes differed between the feather types, indicating that the packing density of the barbules varies (Fig. 1D).
The female also features a wide variety of colours, from green to purplish-red and light brown, but blue is lacking (Fig. 1E, F). The amplitudes of the reflectance spectra measured with the bifurcated probe and microspectrophotometer were rather low compared to the corresponding spectra of the male (Fig. 1G, H). Interestingly, the rump patch of the female measured with the bifurcated probe yielded a double-band reflectance spectrum, with peaks at 560 nm and 670 nm (Fig. 1G). Reflectance spectra measured locally with the microspectrophotometer yielded single-band spectra (Fig. 1H, rump1 and rump2), but double-band spectra could also be measured (Fig. 1H, rump). The latter spectra as well as the double-band probe spectra are due to the summed reflectance from differently structured areas.
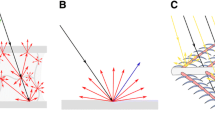
To investigate the spatial distribution of the light reflected by single barbules, we performed scatterometry on feathers from the front-side patches (gorget, crown, and belly) of a male (Fig. 2). When illuminated with a close-to-normal incident white light beam (spot diameter ~ 13 μm), the gorget (g) and crown (c) barbules display a very spatially restricted scatterogram, characteristic for specular reflection (Fig. 2A, B). The belly (b) barbules produced a slightly diffuse pattern, due to some irregularities in the barbule surface (Fig. 2C). Figure 2D shows the scatterogram when the crown barbule of Fig. 2B was illuminated with a very large (wide-angled) illumination aperture. With an increasing angle of incidence and reflection, the colour of the reflected light changes from green (with normal illumination; centre) to blue (with oblique illumination; periphery), which is characteristic for a multilayer reflector.
Scatterograms of feather barbules of Coeligena helianthea (male). A–C Scatterograms of the gorget (g), crown (c), and belly (b) feathers when illuminated with a narrow-aperture beam. D Scatterogram of a crown feather illuminated with a large-aperture beam (cL). The red circles indicate angular directions of 5º, 30º, 60º, and 90º
Structure of the feather barbules and modelled reflectance spectra
The optical experiments indicated that the melanosome stacks act as multilayer reflectors, probably similar to those investigated previously in the male Anna’s Hummingbird (Giraldo et al. 2018). To interpret the measured spectra, we calculated reflectance spectra with the optical modelling approach of the latter study, using parameters following from the anatomy of the barbules of C. helianthea. Transmission electron microscopy revealed that the barbules of the gorget contain a large stack of 10 melanosomes (Fig. 3A), while the belly barbule has 6 melanosome layers (Fig. 3B). The air layer thickness appeared to be the parameter that determines the spectral position, and we therefore calculated the spectra for a few values of that parameter (Fig. 3C; gorget and belly, thin lines) together with the averaged spectrum (Fig. 3C, bold lines).
Anatomy and modelling. A Transmission electron microscopy sections of a gorget barbule of the male C. helianthea. B A belly barbule. C Reflectance spectra obtained with the optical model for melanosome multilayer reflectors of Giraldo et al. (2018). The thickness of the air layers was varied; for the gorget, 80–90 nm, and for the belly, 95–105 nm (thin lines), with an average of the three spectra (bold lines). Scale bar (A, B): 500 nm
Feather reflectance and photoreceptor sensitivity spectra
The feather reflectance spectra appear to be well-separated, similar to the spectral sensitivities of bird photoreceptors. We investigated a possible relationship by calculating the photoreceptor sensitivity spectra of the Blue Tit (Fig. 4A) using data of Hart and Vorobyev (2005). Furthermore, we normalized the reflectance spectra of Fig. 1C for the gorget, crown, and belly (Fig. 4B). We then shifted each of the three reflectance spectra along the abscissa, keeping the spectral shape constant, assuming a constant illumination spectrum, and calculated the effective absorption of the feather reflections by the three corresponding photoreceptors as a function of the reflectance peak wavelength (Fig. 4C). The peak wavelengths of the normalized effective absorption spectra virtually coincided with the reflectance peak wavelengths. This indicates that the feather reflectance spectra are well-tuned to the photoreceptor spectra.
Feather colouration and photoreceptor sensitivity. A Spectral sensitivity of the ultraviolet (UV), short-wavelength (SW), middle-wavelength (MW), and long-wavelength (LW) sensitivity photoreceptors of the Blue Tit (Hart and Vorobyev 2005; Stavenga and Wilts 2014). B Normalized reflectance spectra of the gorget, crown, and belly feathers of Fig. 1C. C Effective light capture by SW, MW, and LW sensitive photoreceptors as a function of the position of the reflectance peak wavelength of the feathers
Discussion
Both the male and female of the studied species, C. helianthea, have a rich set of coloured feathers (Fig. 1). The feathers’ structural colours are created by the stacks of air-filled melanosomes in the barbules (Fig. 2). By treating the melanosome stacks as multilayer reflectors, the measured reflectance spectra can be well-explained. The calculations show that slight changes in the melanosome dimensions cause distinctly different reflectance spectra (Fig. 3). It was hence striking that the different feather reflectance spectra strongly overlap with the set of photoreceptor sensitivity spectra, which suggested that feather and photoreceptor spectra are tuned (Fig. 4).
Our study extends the early modelling work by Greenewalt et al. (1960a) and our more recent analysis of the Calypte anna hummingbird feathers (Giraldo et al. 2018). Here, we furthermore investigated the possible tuning of the sensitivity spectra of the photoreceptors and the feather reflectance spectra. Hummingbirds have four photoreceptor classes (Chen and Goldsmith 1986), like other birds (Hart and Vorobyev 2005). Unfortunately, intracellular recordings of hummingbird photoreceptors have not yet been performed. The Green-backed Firecrown hummingbird, Sephanoides sephanoides, is the only hummingbird species where spectral sensitivity data were measured by electroretinography (Herrera et al. 2008). By fitting modelled responses of one double- and four single-cone types to the measured electroretinograms, photoreceptor peak wavelength values were derived. The short-, middle-, and long-wavelength receptors were thus concluded to have peak sensitivities at 440, 508, and 560 nm, respectively. No errors in the derived peak wavelengths were provided in the Herrera et al. paper, but considering the given measurement errors, the uncertainty in the derived peak wavelengths is very large. More importantly, the reported peak wavelength values are in severe conflict with the minor spread of the peak wavelengths at 463 ± 11, 540 ± 4, and 605 ± 5 nm of the corresponding bird photoreceptors in the large data set reported by Hart and Vorobyev (2005). The photoreceptor spectra concluded for S. sephanoides are strongly hypsochromic shifted and far outside the range of the other related bird photoreceptors. Presumably, the large contribution assumed for the double cone caused the concluded erroneous peak wavelengths.
The peak wavelength of the red receptor concluded for S. sephanoides seemed especially unrealistic, and also since the reflectance spectrum of the red belly feathers peaks at 603 nm, we decided to rely our analysis on the general bird database (Hart and Vorobyev 2005), taking as an example the data of the Blue Tit, filtered by the ocular media (see also Stavenga and Wilts 2014). The photoreceptor and feather reflectance spectra thus appeared to have a striking correspondence (Fig. 4). Tuning of photoreceptor and feather spectra has also been discussed by Wilts et al. (2014). Ornithophilous flowers that are visited by hummingbirds seem not to be tuned specifically to hummingbird colour sensitivity (Altshuler 2006), but tuning of photoreceptor and feather reflectance spectra might be an evolutionary result of courtship and territorial displays, as proposed to hold for other hummingbird species (Simpson and McGraw 2018a, b).
Conclusion
We studied the optical basis of the sexual dichromatism of Coeligena helianthea, a model species for studying phenotypic differentiation in reproductive isolation, and we related the reflectance spectra of the feathers with the spectral sensitivities that had previously been reported for birds. The correspondence of the spectra indicates the crucial role of the feather colouration in sexual communication in this hummingbird species.
References
Altshuler DL (2006) Flower color, hummingbird pollination, and habitat irradiance in four neotropical forests. Biotropica 35:344–355
Bleiweiss R (1985) Iridescent polychromatism in a female hummingbird: is it related to feeding strategies. Auk 102:701–713
Bleiweiss R (1999) Joint effects of feeding and breeding behaviour on trophic dimorphism in hummingbirds. Proc R Soc B 266:2491–2497
Chen DM, Goldsmith TH (1986) Four spectral classes of cone in the retinas of birds. J Comp Physiol A 159:473–479
Dufresne ER, Noh H, Saranathan V, Mochrie SG, Cao H, Prum RO (2009) Self-assembly of amorphous biophotonic nanostructures by phase separation. Soft Matter 5:1792–1795
Durrer H (1977) Schillerfarben der Vogelfeder als Evolutionsproblem. Denkschr Schweiz Naturforsch Ges 91:1–126
Durrer H, Villiger W (1970) Schillerfarben der Stare (Sturnidae). J Ornithol 111:133–153
Endler JA (1993) Some general comments on the evolution and design of animal communication systems. Philos Trans Biol Sci 340:215–225
Endler JA, Westcott DA, Madden JR, Robson T (2005) Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution (NY) 59:1795–1818
Giraldo MA, Parra JL, Stavenga DG (2018) Iridescent colouration of male Anna’s hummingbird (Calypte anna) caused by multilayered barbules. J Comp Physiol A 204:965–975
Greenewalt CH, Brandt W, Friel DD (1960a) Iridescent colors of hummingbird feathers. J Opt Soc Am 50:1005–1013
Greenewalt CH, Brandt W, Friel DD (1960b) The iridescent colors of hummingbird feathers. Proc. Amer. Phil. Soc. 104:249–253
Hart NS, Vorobyev M (2005) Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J Comp Physiol A 191:381–392
Herrera G, Zagal JC, Diaz M, Fernández MJ, Vielma A, Cure M, Martinez J, Bozinovic F, Palacios AG (2008) Spectral sensitivities of photoreceptors and their role in colour discrimination in the green-backed firecrown hummingbird (Sephanoides sephaniodes). J Comp Physiol A 194:785–794
Hill GE, McGraw KJ (2006) Bird coloration, vol I. Harvard University Press, Cambridge (Mass)
Hogan BG, Stoddard MC (2018) Synchronization of speed, sound and iridescent color in a hummingbird aerial courtship dive. Nat Comm 9:5260
Kinoshita S (2008) Structural colors in the realm of nature. World Scientific, Singapore
Meadows MG, Morehouse NI, Rutowski RL, Douglas JM, McGraw KJ (2011) Quantifying iridescent coloration in animals: a method for improving repeatability. Behav Ecol Sociobiol 65:1317–1327
Osorio D, Ham AD (2002) Spectral reflectance and directional properties of structural coloration in bird plumage. J Exp Biol 205:2017–2027
Parra JL (2010) Color evolution in the hummingbird genus Coeligena. Evolution (NY) 64:324–335
Parra JL, Remsen JV, Alvarez-Rebolledo M, McGuire JA (2009) Molecular phylogenetics of the hummingbird genus Coeligena. Mol Phylogenet Evol 53:425–434
Pitelka FA (1951) Ecologic overlap and interspecific strife in breeding population of Anna and Allen hummingbirds. Ecology 32:641–661
Prum RO, Dufresne ER, Quinn T, Waters K (2009) Development of colour-producing beta-keratin nanostructures in avian feather barbs. J R Soc Interface 6:S253–S265
Simpson RK, McGraw KJ (2018a) It’s not just what you have, but how you use it: solar-positional and behavioural effects on hummingbird colour appearance during courtship. Ecol Lett 21:1413–1422
Simpson RK, McGraw KJ (2018b) Two ways to display: male hummingbirds show different color-display tactics based on sun orientation. Behav Ecol 29:637–648
Stavenga DG, Wilts BD (2014) Oil droplets of bird eyes: microlenses acting as spectral filters. Philos Trans R Soc Lond B 369:20130041
Stavenga DG, Leertouwer HL, Pirih P, Wehling MF (2009) Imaging scatterometry of butterfly wing scales. Opt Express 17:193–202
Stavenga DG, Tinbergen J, Leertouwer HL, Wilts BD (2011a) Kingfisher feathers—colouration by pigments, spongy nanostructures and thin films. J Exp Biol 214:3960–3967
Stavenga DG, Leertouwer HL, Marshall NJ, Osorio D (2011b) Dramatic colour changes in a bird of paradise caused by uniquely structured breast feather barbules. Proc R Soc B 278:2098–2104
Stiles FG (1982) Aggressive and courtship displays of the male Anna’s Hummingbird. Condor 84:208–225
Sun J, Bhushan B, Tong J (2013) Structural coloration in nature. RSC Adv 3:14862–14889
Wilts BD, Michielsen K, De Raedt H, Stavenga DG (2014) Sparkling feather reflections of a bird-of-paradise explained by finite-difference time-domain modeling. Proc Natl Acad Sci USA 111:4363–4368
Xiao M, Dhinojwala A, Shawkey M (2014) Nanostructural basis of rainbow-like iridescence in common bronzewing Phaps chalcoptera feathers. Opt Express 22:14625–14636
Acknowledgements
This study was financially supported by the Colombian Administrative Department for Science and Technology—Colciencias (grant code 111571250482), and the Air Force Office of Scientific Research/European Office of Aerospace Research and Development AFOSR/EOARD (grant FA9550-15-1-0068). We thank Prof. Germán Ricaurte from UdeA for reading the manuscript and his useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sosa, J., Parra, J.L., Stavenga, D.G. et al. Sexual dichromatism of the Blue-throated Starfrontlet, Coeligena helianthea, hummingbird plumage. J Ornithol 161, 289–296 (2020). https://doi.org/10.1007/s10336-019-01709-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-019-01709-z