Abstract
A number of studies have shown that migrating birds can navigate to their destinations even when displaced to unfamiliar territory. It has been demonstrated that adult Eurasian Reed Warblers (Acrocephalus scirpaceus) captured in spring in the Eastern Baltic, displaced 1000 km eastward to the Moscow region and tested in orientation cages, show a clear orientation tendency towards their breeding grounds. This response requires the ability to determine a new geographic position relative to the goal. The natural cues that are used as coordinates for this behaviour remain controversial. Among other natural cues, both magnetic and olfactory sources of information have received the most experimental attention. More recently, virtual displacement experiments have shown that the geomagnetic information alone is sufficient for Reed Warblers to find their geographic position. However, the role of olfaction was not explicitly examined. In the present study, we displaced anosmic Reed Warblers together with untreated controls between the same capture and displacement sites where the Emlen funnel tests were previously performed. Following release, we radio-tracked birds for the first few kilometres using an array of automated radio-tracking towers. The results strongly suggest a navigational response of both anosmic and intact birds (anticlockwise re-orientation), unlike some other experiments showing impaired navigational abilities of anosmic migrating birds. This data supports the hypothesis that, at least in this songbird species, the olfactory system is not crucial for determining geographic position, and that the zinc sulfate anosmia treatment is unlikely to have any non-specific effects on navigational abilities.
Zusammenfassung
Anosmische Singvögel kompensieren ihre Zugrichtung nach geografischer Versetzung: eine “radio-tracking“ Studie.
In vielen Studien ist gezeigt, dass Zugvögel in der Lage sind, auch nach geografischer Versetzung in vorher unbekannte Gebiete zu ihren Brutgebieten zu navigieren. So kompensierten im Frühjahr im Kaliningrad-Gebiet gefangene Teichrohrsänger (Acrocephalus scirpaceus), die etwa 1000 Kilometer ostwärts in die Region um Moskau verbracht und nachfolgend in Emlen-Trichtern getestet wurden, für diese Versetzung und zeigten Orientierungsverhalten mit klarer Tendenz zu den nun nordwestlich gelegenen Brutgebieten. Dieses Verhalten impliziert einen Mechanismus, der diese geografische Versetzung relativ zum Ziel detektiert. Die natürlichen Reize, die dafür in Frage kommen, sind umstritten; diskutiert werden magnetische und olfaktorische Reize. Unlängst an Teichrohrsängern durchgeführte virtuelle Versetzungsexperimente deuten darauf hin, dass geomagnetische Reize allein ausreichen, um die geografische Position zu bestimmen. Hierbei wurde jedoch die mögliche Rolle olfaktorischer Reize vernachlässigt. In der vorliegenden Studie wurden anosmische und unbehandelte Teichrohrsänger in gleicher Weise wie in den vorigen Emlen-Trichter Experimenten versetzt, aber diesmal besendert. Nach ihrer Freisetzung wurde die Zugrichtung der Tiere mithilfe von automatischen Radioempfänger registriert. Anders als frühere Untersuchungen, die eine Beeinträchtigung der Orientierung bei anosmischen Zugvögeln zeigten, deuten unsere Ergebnisse klar daraufhin, dass unbehandelte sowie anosmische Teichrohrsänger zu Navigationsverhalten in der Lage sind (Umorientierung entgegen Uhrzeigersinn). Unsere Daten stützen somit die Hypothese, dass der Geruchssinn zumindest in dieser Art eine untergeordnete oder gar keine Rolle bei der Bestimmung der geografischen Position spielt. Zudem zeigt die Studie, dass die Behandlung der Vögel mit Zinksulfat zur „Geruchsblindung“ offenkundig deren Navigationsvermögen nicht beeinträchtigt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of migratory birds to perform their spectacular long-distance journeys and yet precisely locate their breeding or winter grounds has fascinated both scientists and general audiences alike. Displacement experiments have provided strong evidence that birds translocated from their migratory route perform true navigation (Mouritsen 2018). This means that they are capable of identifying and reaching their goal, without direct sensory contact with it, from a place they have never previously visited (e.g., Perdeck 1958; Thorup et al. 2007; Chernetsov et al. 2008; Willemoes et al. 2015; but see Kishkinev et al. 2016). For true navigation, the ability of an animal to determine its current geographic position relative to the goal (a map sense) is crucial. However, the sensory systems and natural cues underlying the map sense for positioning remain poorly understood (Holland 2014; Kishkinev 2015). In this study, we focused on the role of the sense of smell (olfactory system) for long-distance navigation in a migratory songbird species—the Eurasian Reed Warbler (Acrocephalus scirpaceus; hereafter Reed Warblers).
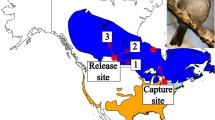
In previous studies (Chernetsov et al. 2008; Kishkinev et al. 2013), it has been established that untreated Reed Warblers on spring migration show a compensatory re-orientation in Emlen funnels (Emlen and Emlen 1966) when displaced 1000 km due east from the south-eastern Baltic (Courish Spit, Kaliningrad region, Russia), to Zvenigorod (Moscow region, Russia). The latter is most probably unfamiliar territory for Reed Warblers migrating through Rybachy according to the ringing recaptures (see Supplementary Material). At the capture site, the birds showed a north-eastern direction, which is in good agreement with the breeding destinations of Reed Warblers further north and northeast in Baltic countries, south Finland and the north-western part of Russia (Supplementary Material). At the displacement site, which is outside their migratory route and to the southeast of their breeding sites, the same birds demonstrated a significant orientation towards the northwest. This result suggested that Reed Warblers were able to determine that they were at a new location and to compensate for the translocation. Which natural cues the Reed Warblers use to be able to determine their displacement to Zvenigorod has been the focus of the subsequent studies (Kishkinev et al. 2010, 2013, 2015; Pakhomov et al. 2018).
A number of cues have been proposed to play a role in the navigational map of birds, but two in particular have retained prominence. The magnetic navigation hypothesis proposes that animals use the Earth’s magnetic field parameters because of their relatively predictable spatial distribution (Phillips 1996; Mouritsen 2018). In many regions of the world, total intensity and inclination (the angle between the magnetic field lines and the horizon) of the geomagnetic field varies approximately along a north–south axis, whereas declination (the angle between directions towards the magnetic and geographic poles) changes primarily along an east–west axis (Boström et al. 2012; https://ngdc.noaa.gov/geomag/WMM/image.shtml). The trigeminal nerve has been shown to be involved in the avian magnetoreception (Heyers et al. 2010; Lefeldt et al. 2014; Elbers et al. 2017), but it is not required for magnetic compass orientation (Zapka et al. 2009). A further displacement experiment from the Courish Spit to Moscow demonstrated that an intact trigeminal nerve is necessary for the compensatory response shown by Reed Warblers (Kishkinev et al. 2013). Following on from this, exposure to a changed magnetic field alone at the capture site simulating the displacement site in Zvenigorod elicited a compensatory goal-ward anti-clockwise re-orientation similar to the one documented after a real geographic displacement (Kishkinev et al. 2015), and these compensatory responses also required intact trigeminal nerves (Pakhomov et al. 2018). Another experiment at this capture site in which only declination was changed to match a location in Scotland showed that this magnetic parameter appears to be used to calculate longitudinal position, at least in Western Europe (Chernetsov et al. 2017). This suggests that the Earth’s magnetic field alone can be sufficient for locating geographic position in Reed Warblers. It is argued, however, that the navigation systems of birds can be redundant and employ various cues (Walcott 1996).
An alternative but not mutually exclusive explanation of the Reed Warblers’ navigation is the olfactory map hypothesis, which proposes that birds make use of olfactory cues to locate their position. Support for this hypothesis comes, in part, from a large number of displacement experiments and other studies on homing pigeons that show reduced homing performance when the pigeons are made anosmic, or reorientation when odours are manipulated (reviewed extensively in Wallraff 2005; Gagliardo 2013). There is also strong evidence from shearwaters and other tubenoses (Procellariiformes) which also showed impaired homing performance when made anosmic (e.g., Gagliardo et al. 2013; Pollonara et al. 2015). Two experiments on very different species with both olfactory and magnetic senses being manipulated have indicated that an intact olfactory system, but not magnetic cues, is necessary for true navigation during migration. Both Gray Catbirds (Dumetella carolinensis; Holland et al. 2009), and in some cases Lesser Black-backed Gulls (Larus fuscus fuscus; Wikelski et al. 2015), seemed to be unable to correct for a displacement when made anosmic, but to be unimpaired when a magnetic treatment was applied.
Given the mismatch between the results of experiments on Reed Warblers and other migratory species tested, and the fact we did not precisely control odours and the sense of smell during our virtual magnetic displacements (Kishkinev et al. 2015; Pakhomov et al. 2018), in this study we aimed to explicitly examine the role of the olfactory sense in the compensatory behaviour of Reed Warblers after displacement, by studying their orientation during free flight after take-off, rather than in Emlen funnels, as free-ranging birds have been used in other studies (Holland et al. 2009; Wikelski et al. 2015). If the magnetic map hypothesis reported above is correct, we expected that the ability to determine their position should remain intact in both control and anosmic birds so that they would be able to compensate for the translocation and show their initial flight directions shifted anti-clockwise, compared to their normal migratory direction (see Fig. 1a for scenarios 2−4). This would be in general agreement with the result from birds tested in Emlen funnels (Chernetsov et al. 2008; Kishkinev et al. 2013, 2015; Pakhomov et al. 2018) and/or with the real movements of displaced Common Cuckoos (Cuculus canorus) according to Willemoes et al.’s (2015) satellite tracking study. If the olfactory system plays a necessary role in true navigation, we predicted that birds without a functional sense of smell would not be able to determine their new location and either show disorientation (departure in random directions) or fall back to the normal migratory direction, as has been seen in other studies (Holland et al. 2009; Wikelski et al. 2015), whereas the control group would adjust to the displacement by shifting their orientation anti-clockwise, as mentioned above.
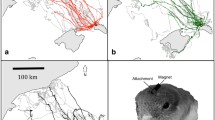
Results of the displacement experiment with free-flying anosmic Eurasian Reed Warblers (Acrocephalus scirpaceus). a The capture site (Rybachy, Kaliningrad region), the displacement site (Zvenigorod, Moscow region) and the breeding range of Eurasian Reed Warblers in the region (shaded grey according to BirdLife International and NatureServe 2012). The thick semi-transparent arrow shows the approximate migratory corridor in spring for birds later captured at Rybachy (Supplementary Material for the ringing data). The dashed line arrow at the capture site shows the mean migratory direction of Eurasian Reed Warblers passing through Rybachy according to the ringing data (Supplementary Material). The dashed broken arrows at the displacement site show our working hypotheses: (1) no compensation and the migratory direction of capture site; compensation (2) towards the centre of breeding destinations (solid line oval based on the ringing recaptures, Supplementary Material), or (3) towards the capture site or (4) towards the migratory corridor in Europe before the capture site (Supplementary Material). b, c The circular diagrams show the orientation of Eurasian Reed Warblers tested in Emlen funnels at the capture site during spring migration 2004–2007 (the data are from Chernetsov et al. 2008). d, f Displacement site with automated radio towers and vanishing directions of free flying birds from anosmic d and control f groups. RS is a release site tower with four antennas oriented in cardinal directions. P1−P3 are three periphery towers with two antennas each. Length of grey antenna segment represent an approximate estimate of their detection range (~ 1 km). Black solid arrows show vanishing direction of birds that departed by identified migratory night-time flights. Long arrows (2−3 km) represent directions of flights detected first at the RS tower and then by periphery towers. Short arrow (1 km) indicate departures recorded only by the RS tower. e, g Circular diagrams showing the departure directions shown on d, f for anosmic (eα = 237°, r = 0.75, n = 10, 95% confidence interval, CI, 205°−268°; P = 0.002) and control birds (gα = 269°, r = 0.89, n = 7, 95% CI 243°−294°; P < 0.001). For all the circular diagrams, each dot at the circle periphery indicates either the mean orientation (b, c) or the vanishing direction (e, g) of one individual bird; arrows show mean group vectors; dashed circles indicate the radius of the group mean vector needed for 5 and 1% levels of significance according to the Rayleigh test of uniformity; solid lines flanking mean group vectors give 95% confidence intervals for the group mean directions. Maps were drawn using R (R Core Team 2017)
Methods
Experimental birds and normal migratory direction
To be consistent with previous displacement experiments in this region (e.g., Chernetsov et al. 2008), we used Eurasian Reed Warblers. This is a common long-distance avian migrant breeding in Europe and overwintering in sub-Saharan Africa (Procházka et al. 2018; Supplementary Material). They migrate alone and at night. The orientation of this species has been tested in Emlen funnels in spring at both capture and displacement sites (Chernetsov et al. 2008; Kishkinev et al. 2013).
For this study, we captured 26 Eurasian Reed Warblers during their spring migration with mist-nets (21−27 May 2016) at Rybachy, on the south-eastern Baltic coast (55°09ʹN, 20°52ʹE). In spring, all birds have gained migratory experience (have performed at least one autumn and a part of spring migration with age from 11 months and older) and possess navigational skills because the latter require migratory experience (Perdeck 1958; Mouritsen 2003; Thorup et al. 2007; Holland 2014). We aimed to preferentially select transit, rather than local, individuals heading further north and northeast towards their breeding sites (Supplementary Material). At the capture site, we selected relatively fat individuals (mean subcutaneous fat score, according to Kaiser 1993, was 2.8, SD ± 1.6) because a previous study based on the Rybachy ringing data reported that 66% of local Reed Warblers arrive with the fat score of 0 as opposed to only 36% in transient birds (Chernetsov 1999). The migratory state of the captured birds was confirmed by the timing of their departures after the displacement (see “Results”). On average, the birds gained subcutaneous fat from the capture date until release (see “Translocation, displacement site and release”). The birds were kept in individual cages (60 × 20 × 20 cm) placed inside an outdoor aviary so that they had a clear view of celestial orientation cues. The cages had good air circulation so that local olfactory information was available before displacement. The captive birds were always exposed to natural light and photoperiod, and provided with food (mealworms) and water with added vitamins ad libitum.
The birds’ orientation was not tested in Emlen funnels at the capture site to allow enough time for habituation to captivity, transportation, post-transportation rest and time for radio-tracking at the displacement site before the end of the spring migratory state (mid-June for this species). Based on the numerous orientation tests of these migrants at the same capture site in 2004−2017 (Chernetsov et al. 2008; Kishkinev et al. 2013, 2015; Pakhomov et al. 2018), there is good reason to assume that the control orientation of transit birds in spring is towards the northeast (Fig. 1b). These Emlen funnel data at the capture site are in good agreement with the ringing recaptures (Supplementary Material).
Experimental groups and treatments
Before displacement, the captured birds were randomly assigned to two equal size groups (n = 13 in each). Given the typically high concentration of free-flying birds’ mean group directions in our previous studies (Holland 2010; Kishkinev et al. 2016), such a sample size should ensure adequate power to determine re-orientation (from the normal north-eastern direction to north-western or due western ones). For anosmia, we used the same zinc sulfate protocol that was previously used in gray catbirds (Holland et al. 2009), Cory’s shearwaters (Gagliardo et al. 2013) and in numerous homing pigeon studies (Wallraff 2005; Gagliardo 2013 for review). The nasal cavities of all the birds were irrigated with two freshly prepared water-based solutions: 4% aqueous zinc sulfate solution (ZnSO4·7H2O) for the anosmic group and saline solution (0.9% NaCl) for the control group. We used fresh and filtered water for the solution preparation. For the nasal cavity treatments, we briefly restrained a bird with the beak fixed in a half-opened position and the head looking down to allow a washing solution to flow out from nostrils and prevent it being swallowed. We filled a syringe with the washing solutions, gently inserted the blunt and bent tip of a needle into the birds’ choanae (left and right side), slowly filled up the nasal cavity and stopped when the solution dripped from the nostrils. We let the solution completely drain after the treatments. All the treatments were performed on the same day (1 June 2016), a day before the translocation and 5−10 days before the departure times at the displacement site. See “Discussion” for the estimated longevity of the zinc sulfate-induced anosmia based on the previous studies.
Translocation, displacement site and release
All the experimental birds were displaced on 2 June 2016, 1004 km due east to Zvenigorod Biological Station (ZBS) of Moscow State University, 40 km west of Moscow (Fig. 1a; 55°42ʹN, 36°45ʹE). The birds were transported inside two plastic cages made of white translucent Plexiglas so that they were mainly exposed to the natural photoperiod (except for one of two boxes that was placed inside a cargo room for the 1.5-h flight). The transportation boxes had individual compartments, with food and water in each during transportation. The translocation was done by air (a direct flight from Kaliningrad to Moscow Airport Sheremetyevo, 1.5 h), then by car directly to ZBS (approx. 2 h). The whole translocation procedure took approximately 6.5 h. All the birds survived the displacement and were immediately placed into individual cages 40 × 20 × 30 cm at a laboratory of ZBS, provided with water and food and given 3 nights of rest. The release was carried out on 5 June 2016, between 1700 and 1950 hours local time (before the sunset at 2106 hours). The weather on the release day was calm, without rain or strong winds. The weather between the day of release and the end of radio tracking was changeable, with periods of clear sky and part cloudiness, winds of moderate speeds (see more details in “Results”) and intermittent short showers.
The release site was in the Moskva river valley, on a small meadow 80 m from the river. This was a flat landscape, covered with a mixture of shrubs and small coniferous trees in which most released birds were sitting until departure, as per the ground radio-tracking results. The river valley was surrounded by a large open field on the northern side and a dense coniferous and deciduous forest on the southern side. There were no large reed beds (typical Reed Warbler’s habitat) near the release site. In similar suboptimal habitat for the species, migrating Reed Warblers tend to stay very locally and depart as soon as possible (Ktitorov et al. 2010). The fat score check immediately before release showed that all birds had gained subcutaneous fat deposits since the day of capture (n = 26, mean score 3.5, SD ± 0.6).
Radio tracking and departure directions
We radio-tracked initial migratory directions of the displaced birds. Initial flight directions are often in good agreement with season-specific migratory directions and show higher concentrations compared to Emlen funnel tests (e.g., Holland 2010; Chernetsov et al. 2011; Holland and Helm 2013; Kishkinev et al. 2016). Each bird was fitted with a 0.6-g Nano Tag NTQB-2 digitally-coded radio transmitters (LOTEK; Newmarket, ON, Canada), with a total weight with harness of < 5% of a bird, using a leg-loop harness (Rappole and Tipton 1991) immediately before release. All the radio transmitters operated at a frequency of 150.300 MHz, and each tag emitted pulses every 4.8 or 5.2 s. The ID of each tag was coded by a unique combination of spacing (time lags) between individual pulses, and each ID was decoded automatically by radio receiver firmware while storing data. The warranted lifespan of the radio transmitters was 14 days.
Bird movements were tracked for 6 days (5−11 June 2016) using four automatic radio towers: one located at the release site (RS tower, 55°42ʹ09.28″N, 36°43ʹ39.23″E) and three additional perimeter towers in Anikovo (55°42ʹ09.19″N, 36°41ʹ38.09″E), Karinskoe (55°42ʹ49.31″N, 36°40ʹ59.67″E), and Rybushkino (55°42ʹ37.88″N, 36°44ʹ01.09″E), situated correspondingly at distances of 2.1, 3 and 1 km to the west, northwest and northeast, respectively, of the RS tower (Fig. 1). For the RS and Anikovo towers, we used SRX800 automatic receivers (LOTEK) that can scan antennas consecutively, one at a time, via a switch box. Our SRX receivers used 8-s scan time per antenna (32 s total scanning time for the RS tower and 16 s for the Anikovo tower). For the Karinskoe and Rybushkino towers, we used Sensorgnome receivers (http://sensorgnome.org; the project underlying the Motus Wildlife Tracking System, https://motus.org, Taylor et al. 2017) that scan all antennas simultaneously. For all the towers, we used the same 3-element Yagi antennas made for 150 MHz. At the RS tower, we used four antennas attached to two wooden poles affixed to the top of a metal meteorological tower at a height of 12 m above the ground. The RS antennas were pointing in the following orthogonal directions spanning 90°: 32°, 122°, 212° and 302°. These antennas allowed us to register vanishing directions during departures from the release site. At the perimeter towers, we attached the antennas to the top of vertical wooden poles so that they were 6−7 m above the ground. Each perimeter tower had two antennas roughly oriented in the direction of adjacent neighbouring towers (Fig. 1d, f). This perimeter tower layout formed almost a full circle around the release site, covering the expected vanishing directions except for the least expected directions in the south to southeast sector (Fig. 1a, d, f). Thus, no matter which migratory direction a released bird would take, its vanishing direction would be registered by either the RS tower or both at the RS and one or more perimeter towers for up to 3−5 km (Fig. 1d, f). Based on our past field measurements and radio-tracking studies with free-flying birds (e.g., Chernetsov et al. 2011), we conservatively assess the detection range of each antenna as approximately 1 km (Fig. 1d, f). It is worth noting that the detection range of Yagi antennas depends on such factors as weather and air humidity, the height of an antenna above the ground, the position of a bird relative to an antenna, local radio interference and properties of electrical components receiving the radio signals. Data for each tower were downloaded at least every second day and on the last day of radio-tracking.
We detected departure events by the initial characteristic spike of the radio signal (a bird starts taking off and elevating above surrounding vegetation) followed by a gradual decrease and disappearance from the RS tower. In some cases, we were able to detect signals both at the release and perimeter tower(s). For departures registered only by the RS tower, we estimated vanishing directions by either approximating it to a bearing of the antenna with the longest signal detected (if signals at the other three antennas stopped simultaneously) or weighting bearings of two antennas with the longest signal detected (the method used in Holland 2010). For departures detected by the RS tower and perimeter tower(s), we estimated the vanishing direction by drawing a vector connecting the release site and the position of perimeter tower or a point at the perimeter tower antennas detection line that fits best to the signal pattern.
For more information about the technical specifications and operation of receivers, as well as the techniques of calculating vanishing directions, see Supplementary Material.
Data analysis and statistics
We processed and visualised radio signal data using custom-written R scripts (R Core Team 2017). For circular data, we used the standard Rayleigh test of uniformity (Batschelet 1981) to assess if a mean group direction significantly differed from a uniform distribution (the null hypothesis). To compare the migratory direction between the control and anosmic groups, circular statistics were performed using Oriana (v.4.02; http://kovcomp.co.uk, Kovach Computer Services, Pentraeth, UK). Differences in mean direction between groups were analysed using the parametric Watson−Williams F test because the assumptions underlying this test (von Mises distribution, the vector lengths r ≥ 0.75, Batschelet 1981) were fulfilled. To test if the mean group directions tended to cluster around the 3 expected goalward-oriented directions (scenario 2—towards the centre of the breeding range with the City of Tallinn, 59°26ʹN, 24°45ʹE, as a proxy destination; scenario 3—towards the capture site at Rybachy, 55°09ʹN, 20°52ʹE; scenario 4—towards the middle point between Rybachy and Gibraltar, which is a proxy of the middle point for the spring migratory corridor inside Europe before the capture site, 46°18ʹN, 5°21ʹE), we used 95% confidence intervals of mean group directions and initial great circle bearings connecting the release site and the above goals (α = 305°, 273° and 257° for the scenarios 2−4, correspondingly; calculated at https://www.movable-type.co.uk/scripts/latlong.html). Differences in mean direction between experimental groups were analysed using comparison of 95% confidence intervals for mean group directions and the parametric Watson−Williams F test in the cases where the assumptions underlying this test were fulfilled. This is automatically tested by the newest version of the circular statistics program, Oriana, v.4.02. For testing the effect of winds on departure directions, we extracted surface wind data for the displacement site for the night hours (2200 and 0100 hours local time, closest to migratory departures) during the radio-tracking period using https://earth.nullschool.net (weather data from GFS, the Global Forecast System).
Results
From 26 birds translocated and released near Zvenigorod (13 anosmic and 13 control), 25 individuals left the detection area of the array within the tracking period of 6 days (05−11 June 2016), mainly during the first post-release night (n = 18, 69% of all the translocated birds). Only one control group bird was still present near the release site on the last day of radio-tracking. Of the 25 individuals that left the release site during the radio-tracking, the departure events of 18 birds (10 anosmic and 8 control) were detected by the radio receivers. These events were typical migratory night-time take-offs near the release site and migratory flights away from the release site. The times of departures tended to cluster in the first half of the night closer to the middle of the night (mean time after sunset 200 min, SD ± 53 min, for anosmic and 149 min, SD ± 59 min, for control). For 4 anosmic and 5 control birds departing by nocturnal migratory flights, we recorded departure signals at both the release site and perimeter towers (Fig. 1d, f long arrows). For 6 anosmic and 2 control birds, we registered departure only at the release site tower (Fig. 1d, f short arrows). For 1 control bird, we detected departure only at a perimeter tower (not taken into directional analysis due to unknown starting site). In total, we obtained the directions of migratory flights for 10 anosmic and 7 control birds.
The mean direction for the anosmic birds was towards the west−southwest [Fig. 1d, e; α = 237°, r = 0.75, n = 10, 95% confidence interval (hereafter 95% CI) 205°−268°; Rayleigh test of uniformity Z = 5.61, P = 0.002] The mean direction for the control birds was due west (Fig. 1f, g; α = 269°, r = 0.89, n = 7, 95% CI 243°−294°; Rayleigh test of uniformity Z = 5.60, P < 0.001). The 95% CIs of the anosmic and control groups overlapped by 25° (28% of the merged 95% CIs of the both groups), and formally there was no significant difference between the mean group directions (Watson−Williams F test, F = 2.76, P = 0.12, df1 = 1, df2 = 15). The 95% CI of the control group includes both the great circle direction (initial bearing) and rhumb-line (loxodrome) towards the capture site (273° and 266°, correspondingly), but the 95% CI of the anosmic group does not include the great circle initial bearing and does includes loxondrome at the edge of the 95% confidence interval, being slightly more shifted towards the south (CI tests, p < 0.05). At the same time, the 95% CIs of the both groups include both 257°, i.e. the great circle direction, and the rhumb-line direction (244°), towards the middle point of the spring migratory corridor inside Europe before the capture site (see Supplementary Material for the spring migratory route of Reed Warblers). Interestingly, the mean directions of both groups differ from the orientation of the captive intact birds tested in Emlen funnels at the same displacement site in 2004−2007 (Fig. 1c; α = 334°, r = 0.41, n = 52, 95% CI 308°−360°; Rayleigh test of uniformity Z = 8.56, P < 0.001): the 95% CIs do not overlap and the Emlen funnel orientation is significantly different from the anosmic and control free-flying groups (Watson−Williams F test, anosmic free-flying birds vs. Emlen funnel tested birds: F = 17.43, P < 0.001, df1 = 1, df2 = 60; control free-flying birds vs. Emlen funnel tested birds: F = 7.28, P = 0.009, df1 = 1, df2 = 57). The prevailing winds during the hours of departure flights were from the north-west but with no significant directional tendency (α = 304°, r = 0.37, n = 12; Rayleigh test of uniformity Z = 1.65, P = 0.19) and were of moderate speeds (mean 2.7 m/s, SD ± 1.09; light breeze on the Beaufort scale).
Discussion
Our present study shows that all the displaced birds departed from the displacement site by a typical (for this species) nocturnal migratory flight. This is in line with the studies of migrating Reed Warblers in spring (Holland 2010), including the data obtained at the same capture site (Bolshakov et al. 2003), and is also in good agreement with a large body of literature on the migratory behaviour of long-distance songbird migrants (e.g., European Robins, Erithacus rubecula: Bolshakov et al. 2007; Northern Wheatears, Oenanthe oenanthe: Müller et al. 2018). This result strongly suggests that the displaced birds were still in a migratory state in spite of being relatively late in the season. The sizes of the groups (10 anosmic and 7 controls) are comparable to past radio-tracking studies on bird orientation and navigation (e.g., Thorup et al. 2007; Holland 2010; Kishkinev et al. 2016). Typically, mean group directions of free-flying birds have relatively high concentrations (r = 0.75 and 0.89 in the present study), which is usually much higher compared to Emlen funnel experiments (e.g., compare Fig. 1b, c and Fig 1e, g). This feature of radio-tracking datasets enables experimenters to apply circular tests with high statistical power.
The mean group directions of the anosmic and control groups were generally westward and were not significantly different. These directions are in agreement with the compensation towards the capture site and/or towards the intermediate point of the spring migratory route in Europe before the capture site (scenarios 3 and 4 in Fig. 1a). Such scenarios are biologically plausible and supported by the satellite-tracking data from free-flying Common Cuckoos after displacement (Willemoes et al. 2015). At the same time, the initial flight directions of our displaced birds were different from the expected north-eastern direction of non-displaced birds, as shown by control Reed Warblers tested previously in Emlen funnels at the capture site (Fig. 1b, c; Chernetsov et al. 2008), and by the ringing data of Reed Warblers (Supplementary Material). This result implies anti-clockwise re-orientation of all the translocated birds, irrespective of the treatment, and the ability to compensate for the displacement by determining their new position in unfamiliar territory, i.e., true navigation performance. As our previous studies have shown, translocated birds with a blocked access to magnetic map information, and thus a disabled navigational sense, tended to be oriented northeasterly, i.e., do not re-orient (Kishkinev et al. 2013; Pakhomov et al. 2018).
These directions (west for control and west−southwest for anosmic birds), however, were different from the north-western mean group direction of the displaced birds tested in Emlen funnels near Zvenigorod in 2004−2007 (Chernetsov et al. 2008). The reason for this discrepancy is unclear, and below we propose some interpretations (the list is not exhaustive). The winds may sometimes drift flying birds, but the winds during the departures were moderate (< 3 m/s) and did not have a strong directional tendency. Therefore, they are unlikely to have a large impact on the vanishing directions. Guiding effects of topographical features might also be hypothesised. Some radar and tracking studies have reported cases where routes of migrating birds were in part shaped by large-scale topographical features, especially by vast ecological barriers (e.g., chains of mountains, shorelines and large rivers: Meyer et al. 2000; Wikelski et al. 2015). The most obvious landmark at the release site was the Moskva river, but it had a width of just 20−30 m and obviously did not represent an ecological barrier for the birds. However, we cannot fully rule out the effect of topography or landmarks around the release site on departure directions, which obviously could not affect the birds moving in Emlen funnels because in these they were not able to see the surrounding landscape. Nevertheless, this guiding effect of the river, if present, was unlikely to override the intended flight directions because the river was oriented southwest to northeast and no eastward departure directions were documented.
The present results, despite following the same protocol, differ from the study on adult anosmic Gray Catbirds on autumn migration in the USA, where the zinc sulfate-treated birds (with similar sample sizes to our study) reverted to the population-specific migratory direction at the capture site as opposed to the intact birds which showed a compensatory tendency following displacement to the east (Holland et al. 2009). Our result is also different from numerous data obtained from homing pigeons, in which the 4% zinc sulfate treatment has often, but not always (e.g., releases at familiar sites: Bingman et al. 1998), led to impaired navigation performance manifested in the high scatter of vanishing directions (first 1−3 km) following displacement, as well as lower homing speeds and return rates of anosmic pigeons compared to controls (Wallraff 2005 for review). Similar effects of anosmia have been reported for Common Swifts (Apus apus) and European Starlings (Sturnus vulgaris) with sectioned olfactory nerves (Fiaschi et al. 1974; Wallraff et al. 1995), and zinc sulfate-treated pelagic birds such as some shearwater species (e.g., Gagliardo et al. 2013; Pollonara et al. 2015). A recent displacement study on Lesser Black-backed Gulls suggested that sectioned olfactory nerves led to impaired navigation performance but only at one of two release sites (Wikelski et al. 2015).
In previous experiments, sectioning of the V1-nerve of Reed Warblers has led to a reversion to the population-specific north-eastern migratory direction after physical displacement to the same site (Kishkinev et al. 2013), and after a virtual magnetic displacement simulating the magnetic conditions at Zvenigorod when birds were tested at the capture site (Pakhomov et al. 2018). The sham surgeries simulating V1 sectioning (control for surgical stress) did not prevent the birds from the compensatory re-orientation (Kishkinev et al. 2013; Pakhomov et al. 2018), which supports the hypothesis that the V1-nerve indeed carries magnetic information from sensory cells (though their location remains unknown: Treiber et al. 2012; Mouritsen 2012). However, an interesting outcome of the present study is that this result does not support the hypothesis that the putative magnetosensory cells associated with the ophthalmic branch of the trigeminal nerve are localised in the olfactory mucosa and could be deactivated or destroyed by the zinc sulfate treatment, which causes necrosis of the tissue in this region (Schlund 1992; Mora et al. 2004). If the trigeminal nerve-dependent magnetic information was blocked by the treatment, we could expect a northeasterly orientation of departing birds similar to the birds with a sectioned V1-nerve tested in Emlen funnels at the same site (Kishkinev et al. 2013).
Could the lack of effect be due to absent or insufficient deactivation of the olfactory receptors in Reed Warblers? Surprisingly, the histological impact of the 4% zinc sulfate treatment and its regeneration period have not been investigated in detail in any bird species, despite the fact that the protocol has been actively used in multiple studies since the 1970s (Wallraff 2005; Gagliardo 2013 for reviews). However, the effects of similar treatments have been extensively studied in other animal taxa, particularly in model mammalian species (especially, in Brown Rat, Rattus norvegicus, and House Mouse, Mus musculus). For example, in Brown Rats, the anatomical study by Smith (1938) reported that irrigation of the nasal cavity with just a few drops of 1% zinc sulfate solution (4% solution in our experiments) rapidly, during the first 2 days, led to a structural loss of the olfactory epithelium. In 7−10 days, he observed complete removal of the olfactory epithelium (sensory, basal and supporting cells), and the full regeneration of the olfactory sensory cells was observed only after 2 months. Matulionis (1975) performed a histological and ultrastructural study on two strains of mice and reported distinctive surface alteration and a massive (~ 50%) decrease of the thickness of olfactory epithelium in the first 4−8 days after the zinc sulfate treatment. Gradual regeneration and reorganisation of the olfactory epithelium was observed over 42 days after the treatment, with full recovery after 72 days (Matulionis 1975). It has also been shown that, in Channel Catfish (Ictalurus punctatus; Cancalon 1982) and Northern Leopard Frog (Rana pipiens; Adamek et al. 1984), zinc sulfate irrigation of the olfactory epithelium with concentrations greater than 3% produced rapid general degeneration of the olfactory epithelium, starting from just a few hours post-treatment. Altogether, the above data strongly suggest that the birds in our experiment had enough time before departure to develop anosmia and loss of sense of smell. The period of functional regeneration has varied across different studies and animal taxa (8−40 days in House Mice, Matulionis 1975; 2−4 weeks in Northern Leopard Frog, Adamek et al. 1984; 3−8 weeks in Channel Catfish, Cancalon 1982). Smith (1938) mentioned that in humans the functional recovery of the sense of smell after 1% zinc sulfate treatment had been reported after 1−2 months. There is very limited physiological literature on the period of functional loss and regeneration in birds. In 4% zinc sulfate-treated Homing Pigeons, the most active period of functional recovery, measured as heart responses in ECG recordings, occurred between the 8th and 20th days post-treatment (Schlund 1992). However, it is worth stressing that we have treated our experimental birds in the same way that led to disrupted navigation performance in Gray Catbirds (Holland et al. 2009), shearwaters (Gagliardo et al. 2013) and in numerous Homing Pigeon studies (Wallraff 2005; Gagliardo 2013). The effect of anosmic treatment in the study by Holland et al. (2009) was observed for up to 24 days after the treatment (21 days until the final bearing in average), and the anosmic Cory’s Shearwaters could not return to their breeding colony after displacement for up to 3 months (Gagliardo et al. 2013). Our birds departed 5−10 days after the zinc sulfate treatment. Therefore, considering the results of the above-mentioned studies with anosmic birds, the Reed Warblers should have been still under the anosmic effect of the treatment at that time. Nevertheless, the authors could not completely rule out the possibility that, even though the same protocol of 4% zinc sulfate treatment was used that led to impaired navigational performance in other avian species, the birds’ sense of smell in our experiment might still be functional at least in some individuals. Given the knowledge gap, we believe the field of bird navigation urgently needs to explicitly address the question of which anatomical and functional consequences the zinc sulfate treatment entails. This question, however, requires a set of anatomical, ultracellular and behavioural studies which were beyond the scope of this work.
In conclusion, our result has shown that the treatment used to make the Reed Warblers anosmic does not support the need for odours and a functional olfactory system for detecting geographic position in our model songbird species, the Eurasian Reed Warbler. Cautiously, we do not generalise this conclusion to all avian taxa or even to all passerine species. This result further corroborates the hypothesis of high variation in navigational mechanisms used by different avian species, and hints at high flexibility of bird navigation processes. This study calls for more consistent investigations of the sensory mechanisms underlying bird navigation when different sensory systems (e.g., magnetic and olfactory senses) are selectively deactivated in the same model bird species using the same displacement designs. Such studies will help us compare different species and reveal patterns in the use of magnetic cues and odours for navigation depending on taxonomy, evolution or ecology of certain taxa. We also recommend detailed histological, ultracellular and functional investigations into the effects of zinc sulfate irrigation on the olfactory system of birds because they have been overlooked compared with other vertebrates.
Data availability
The meta-data and datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adamek G, Gesteland R, Mair R, Oakley B (1984) Transduction physiology of olfactory receptor cilia. Brain Res 310:87–97
Batschelet E (1981) Circular statistics in biology. Academic Press, New York
Bingman VP, Alyan S, Benvenuti S (1998) The importance of atmospheric odours for the homing performance of pigeons in the Sonoran Desert of the southwestern United States. J Exp Biol 201:755–760
BirdLife International and NatureServe (2012) Bird species distribution maps of the world. BirdLife International, Cambridge, UK and NatureServe, Arlington, USA. http://www.birdlife.org/
Bolshakov CV, Bulyuk V, Chernetsov N (2003) Spring nocturnal migration of reed warblers Acrocephalus scirpaceus: departure, landing and body condition. Ibis 145:106–112. https://doi.org/10.1046/j.1474-919X.2003.00128.x
Bolshakov CV, Chernetsov N, Mukhin A, Bulyuk VN, Kosarev V, Ktitorov P, Leoke D, Tsvey A (2007) Time of nocturnal departures in European robins, Erithacus rubecula, in relation to celestial cues, season, stopover duration and fat score. Anim Behav 74:855–865. https://doi.org/10.1016/j.anbehav.2006.10.024
Boström JE, Åkesson S, Alerstam T (2012) Where on earth can animals use a geomagnetic bio-coordinate map for navigation? Ecography 35:1039–1047
Cancalon P (1982) Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell 14:717–733
Chernetsov N (1999) Timing of spring migration, body condition, and fat score in local and passage populations of the Reed Warbler Acrocephalus scirpaceus on the Courish Spit. Avian Ecol Behav 2:75–88. http://zin.ru/journals/aeb/pdf/Chernetsov_1999_2_AEB.pdf. Accessed 09 Jan 2019
Chernetsov N, Kishkinev D, Mouritsen H (2008) A long-distance avian migrant compensates for longitudinal displacement during spring migration. Curr Biol 18:188–190
Chernetsov N, Kishkinev D, Kosarev V, Bolshakov CV (2011) Not all songbirds calibrate their magnetic compass from twilight cues: a telemetry study. J Exp Biol 214:2540–2543
Chernetsov N, Pakhomov A, Kobylkov D, Kishkinev D, Holland RA, Mouritsen H (2017) Migratory Eurasian reed warblers can use magnetic declination to solve the longitude problem. Curr Biol 27:2647–2651.e2
Elbers D, Bulte M, Bairlein F, Mouritsen H, Heyers D (2017) Magnetic activation in the brain of the migratory northern wheatear (Oenanthe oenanthe). J Comp Physiol A 203:591–600
Emlen ST, Emlen JT (1966) A technique for recording migratory orientation of captive birds. Auk 83:361–367
Fiaschi V, Farina A, Ioalé (1974) Homing experiments on swifts Apus apus (L.) deprived of olfactory perception. Monitore Zool Ital 8:235–244
Gagliardo A (2013) Forty years of olfactory navigation in birds. J Exp Biol 216:2165–2171
Gagliardo A, Bried J, Lambardi P, Luschi P, Wikelski M, Bonadonna F (2013) Oceanic navigation in Cory’s shearwaters: evidence for a crucial role of olfactory cues for homing after displacement. J Exp Biol 216:2798–2805
Heyers D, Zapka M, Hoffmeister M, Wild J, Mouritsen H (2010) Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. Proc Natl Acad Sci USA 107:9394–9399
Holland RA (2010) Differential effects of magnetic pulses on the orientation of naturally migrating birds. J R Soc Interface 7:1617–1625
Holland RA (2014) True navigation in birds: from quantum physics to global migration. J Zool 293:1–15
Holland RA, Helm B (2013) A strong magnetic pulse affects the precision of departure direction of naturally migrating adult but not juvenile birds. J R Soc Interface 10:20121047
Holland RA, Thorup K, Gagliardo A, Bisson IA, Knecht E, Mizrahi D, Wikelski M (2009) Testing the role of sensory systems in the migratory heading of a songbird. J Exp Biol 212:4065–4071
Kaiser A (1993) A new multi-category classification of subcutaneous fat deposits of songbirds. J Field Ornithol 64:246–255
Kishkinev D (2015) Sensory mechanisms of long-distance navigation in birds: a recent advance in the context of previous studies. J Ornith 156(Suppl 1):145–161
Kishkinev D, Chernetsov N, Mouritsen H (2010) A double-clock or jetlag mechanism is unlikely to be involved in detection of east−west displacements in a long-distance avian migrant. Auk 127:773–780
Kishkinev D, Chernetsov N, Heyers D, Mouritsen H (2013) Migratory reed warblers need intact trigeminal nerves to correct for a 1000 km eastward displacement. PLoS ONE 8:e65847
Kishkinev D, Chernetsov N, Pakhomov A, Heyers D, Mouritsen H (2015) Eurasian reed warblers compensate for virtual magnetic displacement. Curr Biol 25:R822–R824
Kishkinev D, Heyers D, Woodworth BK, Mitchell GW, Hobson KA, Norris DR (2016) Experienced migratory songbirds do not display goal-ward orientation after release following a cross-continental displacement: an automated telemetry study. Sci Rep 6:37326
Ktitorov P, Tsvey A, Mukhin A (2010) The good and the bad stopover: behaviours of migrant reed warblers at two contrasting sites. Beh Ecol Sociobiol 64:1135–1143
Lefeldt N, Heyers D, Schneider N-L, Engels S, Elbers D, Mouritsen H (2014) Magnetic field-driven induction of ZENK in the trigeminal system of pigeons (Columba livia). J R Soc Interface 11:20140777
Matulionis D (1975) Ultrastructural study of mouse olfactory epithelium following destruction by ZnSO4 and its subsequent regeneration. Am J Anat 142:67–90
Meyer SK, Spaar R, Bruderer B (2000) To cross the sea or to follow the coast? Flight directions and behaviour of migrating raptors approaching the Mediterranean Sea in autumn. Behaviour 137:379–399
Mora CV, Davison M, Wild JM, Walker MM (2004) Magnetoreception and its trigeminal mediation in the homing pigeon. Nature 432:508–511
Mouritsen H (2003) Spatiotemporal orientation strategies of long-distance migrants. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 493–513
Mouritsen H (2012) Sensory biology: search for the compass needles. Nature 484:320–321. https://doi.org/10.1038/484320a
Mouritsen H (2018) Long-distance navigation and magnetoreception in migratory animals. Nature 558:50–59. https://doi.org/10.1038/s41586-018-0176-1
Müller F, Eikenaar C, Crysler ZJ, Taylor PD, Schmaljohann H (2018) Nocturnal departure timing in songbirds facing distinct migratory challenges. J Anim Ecol 87:1102–1115. https://doi.org/10.1111/1365-2656.12821
Pakhomov A, Anashina A, Heyers D, Kobylkov D, Mouritsen H, Chernetsov N (2018) Magnetic map navigation in a migratory songbird requires trigeminal input. Sci Rep 8:11975
Perdeck AC (1958) Two types of orientation in migrating starlings, Sturnus vulgaris, and chaffinches, Fringilla coelebs, as revealed by displacement experiments. Ardea 46:1–37
Phillips JB (1996) Magnetic navigation. J Theor Biol 180:309–319
Pollonara E, Luschi P, Guilford T, Wikelski M, Bonadonna F, Gagliardo A (2015) Olfaction and topography, but not magnetic cues, control navigation in a pelagic seabird: displacements with shearwaters in the Mediterranean Sea. Sci Rep 5:16486
Procházka P, Brlík V, Yohannes E, Meister B, Auerswald J, Ilieva M, Hahn S (2018) Across a migratory divide: divergent migration directions and non-breeding grounds of Eurasian reed warblers revealed by geolocators and stable isotopes. J Avian Biol 49:e01769
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Available at: http://www.R-project.org/
Rappole JH, Tipton AR (1991) New harness design for attachment of radio transmitters to small passerines. J Field Ornithol 62:335–337
Schlund W (1992) Intra-nasal zinc sulphate irrigation in pigeons: effects on olfactory capabilities and homing. J Exp Biol 164:171–187
Smith CG (1938) Changes in the olfactory mucosa and the olfactory nerves following intranasal treatment with one percent zinc sulphate. Can Med Assoc J 39(2):138–140
Taylor PD, Crewe TL, Mackenzie SA, Lepage D, Aubry Y, Crysler Z, Finney G, Francis CM, Guglielmo CG, Hamilton DJ et al (2017) The Motus Wildlife Tracking System: a collaborative research network to enhance the understanding of wildlife movement. Avian Conserv Ecol 12:8
Thorup K, Bisson IA, Bowlin MS, Holland RA, Wingfield J, Ramenofsky M, Wikelski M (2007) Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proc Natl Acad Sci USA 104:18115–18119
Treiber CD, Salzer MC, Riegler J, Edelman N, Sugar C, Breuss M, Pichler P, Cadiou H, Saunders M, Lythgoe M et al (2012) Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 484:367–370
Walcott C (1996) Pigeon homing: observations, experiments and confusions. J Exp Biol 199:21–27
Wallraff HG (2005) Avian navigation: pigeon homing as a paradigm. Springer, Berlin
Wallraff HG, Kiepenheuer J, Neumann MF, Streng A (1995) Homing experiments with starlings deprived of the sense of smell. Condor 97:20–26
Wikelski M, Arriero E, Gagliardo A, Holland RA, Huttunen MJ, Juvaste R, Mueller I, Tertitski G, Thorup K, Wild M et al (2015) True navigation in migrating gulls requires intact olfactory nerves. Sci Rep 5:17061
Willemoes M, Blas J, Wikelski M, Thorup K (2015) Flexible navigation response in common cuckoos Cuculus canorus displaced experimentally during migration. Sci Rep 5:16402
Zapka M, Heyers D, Hein CM, Engels S, Schneider N-L, Hans J, Weiler S, Dreyer D, Kishkinev D, Wild JM et al (2009) Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461:1274–1277
Acknowledgements
The authors are grateful for the assistance of J. Brzustowski, Dr H. Schmaljohann, the staff of the biological station Rybachy and especially thankful to the director Dr N. Chernetsov for help and access to their facilities. We are deeply thankful for the general support of Prof. Valery M. Gavrilov, who allowed us to use the facilities of Zvenigorod Biological Station of Moscow State University. We particularly appreciate the support of the landowners in the Moscow region who allowed us to temporary deploy our radio-tracking equipment on their estates. We are very grateful to Dr Anna Gagliardo (University of Pisa, Italy) for the details of the zinc sulfate treatment. We are grateful to Dr D. Dreyer for helping with the German summary.
Funding
The data collection was funded by the Leverhulme Trust, Research Grant (RPG-2013-288, “The mystery of bird migration: testing hypotheses of true navigation”) to R.H. The data analysis and writing was supported by the Russian Science Foundation grant No 17-14-01147 to D.K.
Author information
Authors and Affiliations
Contributions
Design and methodology: D.K., R.H. Data collection: D.K. with the field assistance of A.A. and I.I. at the displacement site. Data analysis: D.K. Writing original draft: D.K. Writing review and editing: D.K., R.H.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The experiments were conducted in accordance with the national animal welfare legislation of Russia. The permit No. 2016-03 was issued by the responsible regional agency of Kaliningrad region, the Regional Agency for Protection, Reproduction and Use of Animal World and Forests. Additionally, the experiments received local ethical approval by the animal welfare ethics review body (AWERB) of the Bangor University.
Additional information
Communicated by H. Mouritsen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kishkinev, D., Anashina, A., Ishchenko, I. et al. Anosmic migrating songbirds demonstrate a compensatory response following long-distance translocation: a radio-tracking study. J Ornithol 161, 47–57 (2020). https://doi.org/10.1007/s10336-019-01698-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-019-01698-z