Abstract
The immune system is a particularly costly activity that competes with other functions of an organism, such as reproduction, although research is lacking on the importance of environmental factors for the parental investment in offspring immunity. This study examines whether ambient temperature impacts the effect of a sheep red blood cell (SRBC) challenge of the adult female’s immune system on the offspring’s phytohaemagglutinin (PHA) responses and whether this impact differs between the Great Tit and Eurasian Blue Tit. In both studied bird species, offspring wing web swelling was lower after immunisation of the mother (although only in 1 research year) compared with the control (PBS) group of females. A stronger or weaker PHA response of chicks resulted from higher or lower parental investment, respectively. In Eurasian Blue Tits, the nestlings’ PHA responses were positively related to ambient temperature in both experimental groups, while in Great Tits they were positively related to temperature in the control group, but no significant relationship was found in the SRBC nests. This suggests that the impact of ambient temperature is species-specific.
Zusammenfassung
Die Umgebungstemperatur beeinflusst den Effekt einer experimentellen Immunisierung von Kohl- und Blaumeisenweibchen auf die PHA-Antwort ihrer Nachkommen
Das Immunsystem ist besonders energieintensiv und steht in Konkurrenz mit anderen Lebensfunktionen von Organismen, z.B. der Fortpflanzung. Allerdings mangelt es an Untersuchungen zur Bedeutung von Umweltfaktoren für die elterliche Investition in die Immunität der Nachkommen. Diese Studie untersucht, ob die Umgebungstemperatur den Effekt einer Herausforderung des Immunsystems adulter Weibchen mittels roter Blutkörperchen vom Schaf (SRCB) auf die Phytohämagglutinin (PHA)-Antwort der Nachkommen beeinflusst und ob sich dieser Effekt zwischen Kohl- und Blaumeisen unterscheidet. Bei beiden untersuchten Vogelarten wiesen die Nachkommen nach Immunisierung der Mutter eine geringere Schwellung am Flügel auf als die Nachkommen nicht immunisierter Weibchen (Kontrollgruppe, der nur Puffer gespritzt wurde); allerdings war dies nur in einem Untersuchungsjahr der Fall. Die stärkere oder schwächere PHA-Antwort der Küken resultierte also aus einem höheren bzw. niedrigeren elterlichen Aufwand. Bei Blaumeisen stand die PHA-Antwort in beiden experimentellen Gruppen in positivem Bezug zur Umgebungstemperatur, während sie bei Kohlmeisen nur in der Kontrollgruppe positiv mit der Temperatur zusammenhing, nicht jedoch in SRBC-Nestern. Dies deutet darauf hin, dass der Einfluss der Umgebungstemperatur artspezifisch ist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune system is known to be particularly costly and thus is subject to trade-offs with other investments of an organism (Stearns 1992; Kerr et al. 2010; McNamara et al. 2013), especially during resource-demanding periods such as the breeding season. Measuring the strength of the immune response involves challenging animals with a chosen antigen and measurement of the induced response (e.g. Buechler et al. 2009; Boughton et al. 2011; Demas et al. 2011). Generally, injection of non-pathogenic antigens triggers antibody production (although not necessary in the innate immune system), e.g. in domestic poultry (Klasing et al. 1987), Japanese Quails Coturnix coturnix japonica (Fair et al. 1999), Magpies Pica pica (Soler et al. 2003), tits Paridae (Ots et al. 2001), Eurasian Collared Doves Streptopelia decaocto (Eraud et al. 2005) and many others.
Exposure to antigens can affect parental care, as mounting an immune response can negatively affect parental investment into offspring (Bonneaud et al. 2003). In 90% of bird species adults show bi-parental care (Cockburn 2006), but females usually invest more resources in offspring than males (Møller and Birkhead 1993; Cockburn 2006). Females are more involved in care of offspring because they stand to lose a greater initial investment than males; the conventional role of males is sexual selection, which mostly consumes their resources (Trivers 1972; Kokko and Jennions 2008). The way that female parental effort modulates offspring phenotype, in particular their immunity, is complex and remains unclear. Chicks of House Sparrow Passer domesticus mothers vaccinated with Newcastle disease virus (NDV) developed a stronger humoral response, but no effect of female injections on the response to phytohaemagglutinin (PHA) of chicks was found (Broggi et al. 2016). Female House Wrens Troglodytes aedon treated with lipopolysaccharide (LPS) produced heavier offspring with a stronger PHA response (Bowers et al. 2012). Exposure of female Tree Swallows Tachycineta bicolor to sheep red blood cells (SRBC) had no effect on the cell-mediated immunity in their broods (Lozano and Ydenberg 2002). In Great Tits Parus major, the PHA responses of nestlings of SRBC-challenged mothers were lower than in control chicks (Grzędzicka 2017).
The immune response of birds may depend on the external environment, e.g. food access. For example, some previous studies found a direct relationship among the food intake, body mass and PHA response (Yellow-legged Gulls Larus cachinnans: Alonso-Alvarez and Tella 2001; Great Tits: Grzędzicka 2017). However, studies describing the importance of environmental factors for the maternal investment in offspring immunity are lacking. This is a serious oversight, because parental care is flexible, changing in response to environmental conditions and the needs of offspring (Rehling et al. 2012). How well parents can mitigate negative environmental effects on their offspring depends on particular ecological pressures in the environment. This is probably a mechanism for coping with temperature anomalies and the challenges associated with decreasing food availability (Auer and Martin 2017). For example, in Tree Swallows parents injected with PHA during cold weather (and at low abundance of aerial insects) showed suppressed immune responses compared to birds treated during more favourable conditions. Therefore, there was an effect of ambient temperature (and food abundance) on the parental immune response (Lifjeld et al. 2002).
Under high temperatures, animals have a weaker immune defence (e.g. Roth et al. 2010; Seppälä and Jokela 2011), because most of them are adapted to a certain temperature range. Immune responsiveness of birds may also be affected by ambient temperature (Lienart et al. 2014). In one of the articles, the authors showed that in the case of Great Tits and Eurasian Blue Tits Cyanistes caeruleus living in central Poland, the extremes of mean temperature in May were: 12.5 °C noted in 2010 (cold extreme) and 15.3 °C in 2012 (warm extreme) (Bańbura et al. 2013). Ambient temperature may affect the metabolic rate if it is higher than the higher critical temperature or lower than the lower critical temperature (Scholander et al. 1950). The lower critical value of the air temperature for the Eurasian Blue Tit is 15 °C (Haftorn and Reinertsen 1985), while its higher thermoneutral zone value is 30 °C (Nager and Wiersma 1996). The Great Tit's thermoneutral zone range is also 15–30 °C (Broggi et al. 2005; Mathot et al. 2016). At low temperatures, the basal energy of a bird becomes more costly as the ambient temperature causes body heat loss (Reinertsen 1983). In addition, high temperatures affect birds' immune function by reducing antibody production (Mashaly et al. 2004).
Negative effects of poor conditions on offspring phenotypes differ among species (Auer and Martin 2017). In our research we chose two short-lived species: the Great Tit and Eurasian Blue Tit, in which both parents take care of offspring (Giudici et al. 2010; Ramírez et al. 2010; Sonerud et al. 2013). Although these species have similar breeding ecology and similar rates of extra-pair paternity, they differ in two aspects of reproductive strategy that may impact their parental investment and potentially also their immune responsiveness during breeding season. Eurasian Blue Tits are facultatively polygynous (Amrhein et al. 2008) and in the researched area have a short breeding season with almost no second broods (own observations), whereas Great Tits are monogamous (Amrhein et al. 2008) and in the described population often raise second broods (Zając 1999; own observations). Great Tit males continue singing during the incubation period, while Eurasian Blue Tit males reduce singing activity after the first days of egg-laying by the female (Amrhein et al. 2008). Moreover, the Great Tit is superior to the Eurasian Blue Tit in the competition for nesting sites (Alatalo 1982; Dhondt and Adriaensen 1999). This may also affect the immune responses and parental effort of birds—if the Great Tit wins the competition, nest boxes can occupy both birds with a stronger and weaker immune system and effort, while in the case of the Eurasian Blue Tit, nest boxes may be occupied generally by stronger birds, selected by competition.
In this article, we examine whether ambient temperature impacts the effect of challenge of the adult female’s immune system on the offspring’s PHA responses and whether this impact differs between the Great Tit and Eurasian Blue Tit. We chose sheep red blood cells (SRBC) for maternal treatment, as it is an antigen that triggers a relatively strong immune response, as shown by the metabolic cost of immune system activation with this antigen (Hasselquist and Nilsson 2012). Specifically, we tested three hypotheses: (1) because the female response to immune challenge is affected by ambient temperature, we expect that the chick response to PHA is related to temperature differently in nests provisioned by SRBC-treated females compared to control nests (interaction: experimental treatment × temperature); (2) the effect of temperature on the chick PHA response is affected by SRBC immunisation and differs between the two tit species (experiment × species × temperature); we predict that the impact of temperature is lower in the case of Eurasian Blue Tits, as they probably invest much effort in a single breeding attempt, especially in population living in the nest boxes that should be occupied mostly by birds strong enough to compete with Great Tits; (3) the effect of female SRBC treatment on the chick PHA response is different in each year of the study (interactions: experiment × year; experiment × species × year), since each breeding season is characterised by specific spring temperatures, food access and so on.
Materials and methods
The Great Tit Parus major and the Eurasian Blue Tit Cyanistes caeruleus are both small passerines of the Paridae family. This study was conducted during 2 years, 2009 and 2011, in the populations of tits living in a nest box study area in the Niepołomice Forest (near Kraków, southern Poland). The study area consisted of 250 nest boxes. The distance between trees with nest boxes was about 40–50 m; nest boxes were hung about 2 m above the ground. The study area is a deciduous forest dominated by an Oak-Hornbeam community, including Oaks Quercus robur and Q. petrea, the Small-leaved Lime Tilia cordata, the European Hornbeam Carpinus betulus and the European Beech Fagus sylvatica. Temperature data for the Niepołomice Forest were obtained using the weather station with the computer programme WeatherLink 5.9.3 (both produced by Davis Instruments; station measurement accuracy: ± 0.2 °C). To investigate the effect of temperature, average daily temperature (°C) was used. In 2009, the average temperature in May was mean = 13.6 °C ± SE = 0.52, and average temperatures in 10-day intervals were: 1–10.05: 13.1 °C ± 0.68; 11–20.05: 13.5 °C ± 1.09; 21–30.05: 14.2 °C ± 0.93. In 2011, the average temperature in May was higher than 2 years earlier, 15.1 °C ± 0.82, while mean temperatures in May in 10-day intervals were as follows: 1–10.05: 10.6 °C ± 1.17; 11–20.05: 16.7 °C ± 0.97; 21–30.05: 18.0 °C ± 0.86.
Field procedures
The study was conducted only on the first broods. From the beginning of April, all nest boxes were checked once a week to determine the laying date, clutch size and hatching success. Hatching date (May 1 = day 1) was the start date for subsequent procedures in a given nest; days of subsequent procedures were denoted as a “+” followed by the number of days from the hatching date. Three days after hatching (+3), breeding females from randomly selected nests were captured using ornithological mist nets to carry out the injection procedure. Both experimental and control nests were located in older and younger forest patches and across various hatching dates. On the day of female injection (+3), parents were ringed for further identification. Sexes of adult birds were determined by the width of the breast stripe (Great Tits), the intensity of the blue colour on the head and wings (Eurasian Blue Tits), biometric measurements and absence/presence of the brood patch.
Females from the experimental group were injected with 0.1 ml 2% dilution of sheep red blood cells (SRBC) in phosphate buffered saline (PBS) (procedure according to Deerenberg et al. 1997; Ros et al. 1997). SRBC causes both an innate and acquired (mainly humoral) immune response (Deerenberg et al. 1997; Cichoń et al. 2001; Ots et al. 2001; Hawley et al. 2005). The theoretical peak of the female humoral response was expected 6 days after injection, at 9 days post-hatching (Snoeijs et al. 2007). In the control group, females were injected with 0.1 ml PBS. Injection was performed intraperitoneally using an insulin needle. The dose (2 mg of SRBC antigen in the injected 0.1 ml) was the same for both species of tits, even though they differ in body mass—in the Niepołomice Forest the Eurasian Blue Tit weighs 11–13 g, while the Great Tit is heavier (14–20 g; personal observation). The doses of sheep red blood cells used by other authors were varied (Table 1). The selected dose was already used for light birds, such as Zebra Finches Taeniopygia guttata (Verhulst et al. 2005), similar in mass to Eurasian Blue Tits. However, also slightly higher doses of 2% SRBC dilution were used for example in the Japanese Quails (Grindstaff et al. 2005) and Black-headed Gulls Chroicocephalus ridibundus (Ros et al. 1997), which are much heavier than Great Tits (Table 1). In this work, the data set consisted of N = 33 Great Tit nests (N = 15 in 2009, N = 18 in 2011) and N = 35 Eurasian Blue Tit nests (N = 13 in 2009, N = 22 in 2011). SRBC was injected to N = 38 females (N = 18 Great Tits and N = 20 Eurasian Blue Tits). The number of control nests in the two study years was N = 15 nests per species (N = 30).
Based on the collected data on daily temperatures during the breeding season, we recorded the average of the daily temperatures during the 4 days before the expected peak of the humoral immune response of the female (+6, +7, +8, +9), to obtain a representative measure of temperatures throughout the period of growing immune response. We assumed that temperature on the day with maximum response of the female could be accidentally different from the average of the previous days, so we used a mean daily temperature of 4 days, during which the female immune response grew. Eleven days after hatching (+11), we measured the body mass of offspring (just before the PHA injection) and T cell-mediated immune responses.
Measurement of the T-cell-mediated immune response
The cell-mediated immunity is commonly measured by the skin-swelling test and a plant lectin, phytohaemagglutinin (PHA), which is widely used in ecological research (e.g. Kennedy and Nager 2006; Martin et al. 2006; Vinkler et al. 2010). The wing web swelling after PHA injection reflects the efficiency of cell-mediated immunity (Smiths et al. 1999; Tella et al. 2002; Martin et al. 2006; Biard et al. 2015) and has been shown to be a condition-dependent trait (Saino et al. 1997; Brinkhof et al. 1999; Moreno et al. 1999; Hawley et al. 2005), which predicts survival (Christe et al. 2000). Although this is a common method, studies on House Sparrows Passer domesticus (Martin et al. 2006) and Scarlet Rosefinch Carpodacus erythrinus (Vinkler et al. 2012) have shown that a response to PHA is more complex than had been previously thought: not only does it correlate with the T-cell-mediated immune response, but it is also connected to innate and adaptive immune functions.
Eleven days after hatching (+11), nestlings were ringed, weighed and injected with 0.2 mg PHA dissolved with 0.04 ml PBS (Sigma, L8754) in the right wing web (procedure according to: Brinkhof et al. 1999; Smiths et al. 1999). The response to PHA was measured as the change in wing web thickness 24 h after injection. Wing web thickness was measured with a pressure-sensitive specimeter (Mitutoyo 7313) by the same person. “Before” and “after” measurements were taken three times from a chick and average values from a given bird were used. Six nestlings were chosen from every nest: the two heaviest, two medium weight and two lightest. In nests of Great Tit in 2009, the average number of chicks (+11) was 10.2, so 6 chicks per nest selected for PHA-treatment were about 59% of the brood; in 2011 the mean number of chicks was 10.6, so on average 57% of Great Tit chicks were chosen. In 2009, the average number of Eurasian Blue Tit chicks in the nest (+ 11) was 11.2, so 6 chicks per nest selected for injections were 54% of the brood; in 2011 the mean number of Eurasian Blue Tit chicks was 10.5, so on average 57% of chicks was immunised with PHA.
Statistical analyses
To test for the effect of experimental treatment, species and ambient temperature on the offspring’s PHA response, a general linear mixed model (GLMM) was applied in JMP 8 for both species. Before running the model, data were checked for normality using the Kolmogorov-Smirnov test in JMP. The distribution of the PHA response (wing web swelling) of nestlings did not deviate from normality in 2009 (D = 0.060; P = 0.150) and in 2011 (D = 0.128; P = 0.078). The PHA response of individual nestlings was used as the response variable, while “nest ID” was treated as a random effect. The nominal explanatory variables were experimental group (PBS-1, SRBC-2), year (2009–11, 2011–2), species (Great Tit-1, Eurasian Blue Tit-2), while continuous variables were mean ambient temperature before the expected peak of the female immune response, nestling body mass and the number of nestlings (on nestling day +11). Interactions among ambient temperature, female experimental treatment, species and year were also included. The estimation method used in GLMM was the restricted maximum likelihood (REML). The power of the performed test and the least significant number (LSN), which are given for each explanatory variable, were estimated in JMP using effect sizes and standard errors from the sample data (see Thusius et al. 2001).
The GLMM model was followed by Tukey’s HSD post hoc comparison tests calculated in JMP to check for details on how the interactions “exp. × year” or “exp. × species × year” influenced the dependent variable when they were significant. For significant interactions between experimental treatment and ambient temperature, we tested the effect of temperature in additional GLMM models, separately in the experimental and the control group in each species, with the PHA responses of individual chicks as the dependent variable, “nest ID” as a random factor and one continuous explanatory variable: the mean temperature during the 4-day period preceding the peak of humoral response.
Results were considered significant if P < 0.05.
Results
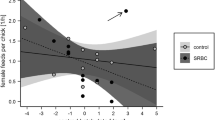
In the main GLMM model, the interaction among temperature, species and experimental treatment was statistically significant and its power was higher than that of significant interaction between the experimental treatment and temperature (Table 2). In the control groups, the nestlings’ PHA response was positively related to ambient temperature during the period preceding the expected female immune response (+ 6, + 7, + 8, + 9) in both the Great Tit (additional GLMM model: R2 = 0.672; estimate = 0.906; SE = 0.274; F = 2.143; df = 1; P = 0.002; power = 0.859; LSN = 28.96; Fig. 1) and Eurasian Blue Tit (GLMM: R2 = 0.603; estimate = 0.036; SE = 0.012; F = 9.394; df = 1; P = 0.009; power = 0.571; LSN = 12.86; Fig. 1), while in SRBC-challenged nests this relationship was non-significant in the Great Tit (GLMM: R2 = 0.569; estimate = 0.015; SE = 0.009; F = 2.463; df = 1; P = 0.130; power = 0.194; LSN = 750.84; Fig. 1) but was positive in the Eurasian Blue Tit, although in this case fit and power of the test were relatively low (GLMM: R2 = 0.138; estimate = 0.013; SE = 0.005; F = 6.351; df = 1; P = 0.013; power = 0.350; LSN = 357; Fig. 1).
The interaction between species, experiment and year was statistically significant, although more than twice weaker than the interaction between experimental treatment and year (Table 2). In the case of the Great Tit, SRBC injection of a female did not decrease chick wing web swelling in 2009 (Tukey’s HSD test, P = 0.542; SRBC: mean = 0.51 ± SE = 0.03; PBS: 0.54 ± 0.04), but it did so in the warmer (in May on average by 1.5 °C) year 2011 (Tukey’s HSD test, P < 0.0001; SRBC: 0.58 ± 0.02; PBS: 0.80 ± 0.02). In the Eurasian Blue Tit, the year effect on the experimental treatment was similar—immune challenge of a female did not affect the offspring’s PHA response in 2009 (Tukey’s HSD test, P = 0.373; SRBC: 0.45 ± 0.03; PBS: 0.49 ± 0.04), but it reduced nestling wing web swelling in 2011 (Tukey’s HSD test, P < 0.0001; SRBC: 0.58 ± 0.02; PBS: 0.89 ± 0.02). In both species, the chicks’ immune response to PHA was lower in both the control and SRBC group in the first year than in the control nests in 2011. Mean differences between 2 years of research in the PHA responses of nestlings from SRBC nests were 0.07 mm in the Great Tit (about 12.8% of the nestlings’ mean mass from 2 years) and 0.13 mm in the Eurasian Blue Tit (25% of the mean mass). In the control nests, PHA responses of the Great Tit nestlings were higher in 2011 than in 2009 for about 0.26 mm (38.8% of the mean offspring mass from 2 years). In the Eurasian Blue Tit, the offspring’s PHA responses were about 0.40 mm (58%) higher in 2011 compared to those from year 2009.
Discussion
In this study, we demonstrated that an immune challenge in Great Tit and Eurasian Blue Tit females with a novel antigen (sheep red blood cells, SRBC) yielded different results on the offspring’s PHA response in the 2 years of study and in the two bird species. In 1 year of the study, in both studied species, the immune challenge of adult females weakened the offspring’s PHA response, consistent with previous research (e.g. Råberg et al. 2000). However, the finding that in both studied species SRBC immunisation of adult females was dependent on the year of study is a novel result, not reported previously.
In 2009 with the lower ambient temperature (mean in May: 13.6 °C)—in contrast to 15.1 °C in 2011, which is comparable to the warm extreme 15.3 °C from 2012 (Bańbura et al. 2013)—the experimental SRBC treatment did not cause differences in PHA responses of nestlings between the two experimental groups. Higher PHA response of chicks in 2011 with higher mean temperature in May can be associated with greater parental effort in the development of the immune system of offspring, suggesting that the PHA response of chicks may be treated as an indicator of the parents' physiological stress. The temperature dependence of the immunisation effect found in our work is a novel result, consistent with general findings that a trade-off between immune defence and reproduction is expected to be more pronounced under harsher environmental conditions (Bonneaud et al. 2003; Ardia 2005). This is true if we assume that the above-mentioned ambient temperature in 2011 is an example of the harsher weather conditions. This result suggests that the PHA response is the adaptive component of immunity, as was reported in a few recent studies (Martin et al. 2006; Vinkler et al. 2012). Our results may also suggest that with higher ambient temperature and probably lower energy expenditure, adult females needed fewer resources for their organisms, so more resources remained for the offspring. Therefore, when the adult female bore the additional cost of the immune response to SRBC injection under high temperatures in 2011, she could have more resources for the development of the chick immune response than under the lower temperatures in the year 2009.
Higher parental investment in the offspring’s PHA response at a higher temperature is in line with the fact that immune response may be compromised by adverse weather conditions (Christe et al. 2001; Ardia 2005; Garvin et al. 2006), although previous works point to the opposite relationship: high temperature affects the immune function by reducing antibody production (e.g. Mashaly et al. 2004). However, adult birds in year 2009 could also invest less in the immune response of their offspring under lower ambient temperatures in that year. We cannot rule out that the ambient temperatures in 2009—which were below the lower value of the thermoneutral zone range of the Great Tit (Broggi et al. 2005; Mathot et al. 2016) and Eurasian Blue Tit (Haftorn and Reinertsen 1985)—could be the factor deteriorating the offspring’s PHA response in that year, regardless of the experimental group (including control). It is known that birds challenged with antigen during lower temperature may also show a suppressed immune response (Lifjeld et al. 2002), which we probably noted in the case of nestlings’ PHA responses in 2009. The aspect of the females’ responses to SRBC is speculative in this case, as we did not measure the antibody titres in the adult females.
In this study, we show that the effect of temperature by SRBC treatment interaction on offspring immune response is species-specific. In the Great Tit, the correlation between nestling PH response and the average temperature during the 4 days when the female was expected to reach the peak of humoral immune response was significant and positive in the control group, but became non-significant in the SRBC-treated group. In the Eurasian Blue Tit, temperature prior to the peak of the female’s humoral response had a significant and positive effect on the offspring cell-mediated response in both experimental groups. The effect of maternal SRBC challenge on the offspring’s PHA response was dependent on ambient temperature only in the Great Tit.
The positive dependence of wing web swelling and temperature in the Eurasian Blue Tits’ nests with the saline-injected female, as well as in those with SRBC injection, may indicate that the pairs of this species occupying nest boxes were strong enough to invest in their offspring survival at a similar level in both experimental groups. The differences between the species can be also explained by differences in their reproductive strategies noted in the populations of the Niepołomice Forest. The Great Tit can breed for the second time in 1 year, so while incurring unforeseen costs (in this study: after SRBC immunisation), it does not invest maximally in PHA response of the chicks in the first brood. Eurasian Blue Tit usually has only one brood per year, so even responding to SRBC it invests in the immune system of the offspring. The investment in the chicks’ PHA response applies not only to the female, but also to the male. We do not know the role of males in the probably modified parental investment after the immunisation of the female, but we cannot exclude that the male can compensate for the increased immune response of the female partner, as was shown previously by other authors (e.g. Great Tits: Hinde 2006; David et al. 2015; Eurasian Blue Tits: Tripet and Richner 1997).
Differences between species may result from the fact that both adults and young birds of both species received the same amounts of antigens, although they differ in size and weight. In the case of Eurasian Blue Tit, the mass of an adult bird in the Niepołomice Forest is usually 11–13 g (own observations), and the average mass of the nestlings chosen for this work is mean = 11.2 g ± SE = 0.21 (on nestling day + 11). For the Great Tit, the mass of an adult bird in the researched population is usually 14–20 g (own observations), while the average mass of the hatchling is 15.2 g ± 0.16 (+ 11). This means that the mass of the adult Eurasian Blue Tit is about 70% of the mass of the Great Tit, and the mass of the Eurasian Blue Tit chick is about 74% of the young Great Tit’s mass. Therefore, the proportion of the dose per unit of body mass used in this work was higher in adults and young Eurasian Blue Tits than for Great Tits, so we should expect the more intensive reactions in the smaller species to both immunisations. It seems that Great Tit females should be injected with about 0.14 ml of 2% SRBC (containing 2.8 mg of this antigen), while nestlings with about 0.27 mg of PHA. Unequal proportions of antigen doses in the birds’ body mass may explain why the offspring’s PHA responses were positively correlated with ambient temperature in both experimental groups of Eurasian Blue Tits: this may be due to stronger parental stress and investment in offspring under the influence of a larger dose compared to that in Great Tits.
On the other hand, the response of adult females to SRBC was not detected in the differences in the offspring’s PHA responses between the two experimental groups in any of the researched species in 2009, despite the different proportions of doses to their body mass. It is also worth noting that the research was carried out on birds that occupy nest boxes with inlets intended for larger Great Tits, so the Eurasian Blue Tits inhabiting those nest boxes could be selected, stronger and thus reacting only to a slightly higher dose than Great Tits (although this is a speculation). Moreover, similar or slightly higher doses of SRBC compared to that in this work were previously used by other authors on various species of birds, even those many times larger than the generally small tits (Table 1). It is not known whether different species of birds have different or similar sensitivity to the injection of sheep red blood cells and phytohaemagglutinin.
In our study, we did not measure the antibody concentration in females responding to the SRBC antigen. So far, it is not known whether an animal producing more antibodies has a stronger immune system or an individual with a more efficient immune system has rather a lower antibody concentration because it is so strong that it can tolerate antigens. Short-lived birds can “down-regulate” their immune response during the breeding season (Deerenberg et al. 1997; Lozano and Ydenberg 2002), but it is still not known which specific environmental conditions are needed for that. Therefore, it is difficult to say whether a short-lived bird, which potentially suspends its immune response during reproduction, is stronger while producing antibodies (because it has resources for immune response and reproduction) or the one that does not respond to the antigen during the energy-demanding period is stronger.
In sum, our results indicate that the cell-mediated immune response of offspring is positively affected by ambient temperature but this relationship is affected by immunisation of the female parent. The effect of the SRBC challenge of adult females on the PHA response of nestlings depends on the ambient temperature in the Great Tit, which partially supports hypothesis 1. We found a difference between the Great Tit and the Eurasian Blue Tit in the interaction effect of ambient temperature and female SRBC challenge on the chicks’ PHA response, which confirms hypothesis 2 or results from the different proportions of the antigen doses per unit of body mass in both bird species. In both studied species we also showed a strong year effect on the interaction effect of temperature and female SRBC treatment on chick PHA response, which supports hypothesis 3.
References
Alatalo RV (1982) Multidimensional foraging niche organization of foliage-gleaning birds in northern Finland. Ornis Scand 13:56–71
Alonso-Alvarez C, Tella JL (2001) Effects of experimental food restriction and body-mass changes on the avian T-cell mediated immune response. Can J Zool 79:101–105
Amrhein V, Johannessen LE, Kristiansen L, Slagsvold T (2008) Reproductive strategy and singing activity: blue tit and great tit compared. Behav Ecol Sociobiol 62:1633–1641
Ardia DR (2005) Tree swallows trade off immune function and reproductive effort differently across their range. Ecology 86:2040–2046
Auer SK, Martin TE (2017) Parental care mitigates carry-over effects of poor early conditions on offspring growth. Behav Ecol 28(4):1176–1182
Bańbura J, Skwarska J, Bańbura M, Glądalski M, Hołysz M, Kaliński A, Markowski M, Wawrzyniak J, Zieliński P (2013) Spatial and temporal variation in heterophil-to-lymphocyte ratios of nestling passerine birds: comparison of blue tits and great tits. PLoS ONE 8(9):e74226
Biard C, Monceau K, Motreuil S, Moreau J (2015) Interpreting immunological indices: the importance of taking parasite community into account. An example in blackbirds Turdus merula. Meth Ecol Evol 6:960–972
Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–379
Boughton RK, Joop G, Armitage SAO (2011) Outdoor immunology: methodological considerations for ecologists. Funct Ecol 25:81–100
Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, Sakaluk SK (2012) Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon). Proc R Soc Lond B 279:2891–2898
Brinkhof MWG, Heeb P, Kolliker M, Richner H (1999) Immunocompetence of nestlings great tits in relation to rearing environment and parentage. Proc R Soc Lond B 266:2315–2322
Broggi J, Hohtola E, Orell M, Nilsson J-Å (2005) Local adaptation to winter conditions in a passerine spreading north: a common garden approach. Evolution 59:1600–1603
Broggi J, Soriguer RC, Figuerola J (2016) Transgenerational effects enhance specific immune response in a wild passerine. Peer J 4:e1766
Buechler DM, Encinas-Viso F, Petit M, Vezina F, Tieleman BI, Piersma T (2009) Limited access to food and physiological trade-offs in a long-distance migrant shorebird. II. Constitutive immune function and the acute-phase response. Physiol Biochem Zool 82:561–571
Christe P, Møller AP, Saino N, de Lope F (2000) Genetic and environmental components of phenotypic variation in immune response and body size of a colonial bird, Delichon urbica (the house martin). Heredity 85:75–83
Christe P, de Lope F, Gonzalez G, Saino N, Møller AP (2001) The influence of environmental conditions on immune reponses, morphology and recapture probability of nestling house martins (Delichon urbica). Oecologia 126:333–338
Cichoń M, Dubiec A, Chadzińska M (2001) The effect of elevated reproductive effort on humoral immune function in collared flycatcher females. Acta Oecol 22:71–76
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc Lond B 273:1375–1383
Dabbert CB, Lochmiller RL, Teeter RG (1997) Effects of acute thermal stress on the immune system of the Northern bobwhite (Colinus virginianus). Auk 114(1):103–109
David M, Pinxten R, Martens T, Eens M (2015) Exploration behavior and parental effort in wild great tits: partners matter. Behav Ecol Sociobiol 69:1085–1095
Deerenberg C, Arpanius V, Daan S, Bos N (1997) Reproductive effort decrease antibody responsiveness. Proc R Soc Lond B 264:1021–1029
Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol 80:710–730
Dhondt AA, Adriaensen F (1999) Experiments on competition between great and blue tit: effects on blue tit reproductive success and population processes. Ostrich 70:39–48
Eraud C, Duriez O, Chastel O, Faivre B (2005) The energetic cost of humoral immunity in the collared dove, Streptopelia decaocto: is the magnitude sufficient to force energy-based trade- offs? Funct Ecol 19:110–118
Fair JM, Hansen ES, Ricklefs RE (1999) Growth, developmental stability and immune response in juvenile Japanese quails (Coturnix coturnix japonica). Proc R Soc Lond B 266:1735–1742
Garvin JC, Abroe B, Pedersen MC, Dunn PO, Whittingham LA (2006) Immune response of nestling warblers varies with extra-pair paternity and temperature. Mol Ecol 15:3833–3840
Giudici A, Navarro J, Juste C, González-Solís J (2010) Physiological ecology of breeders and sabbaticals in a pelagic seabird. J Exp Mar Biol Ecol 389:13–17
Grindstaff JL, Demas GE, Ketterson ED (2005) Diet quality affects egg size and number but does not reduce maternal antibody transmission in Japanese quail Coturnix japonica. J Anim Ecol 74:1051–1058
Grzędzicka E (2017) Immune challenge of female great tits at nests affects provisioning and body conditions of their offspring. Acta Ethol 20:223–233
Haftorn S, Reinertsen RE (1985) The effect of temperature and clutch size on the energetic cost of incubation in a free-living blue tit (Parus caeruleus). Auk 102:470–478
Hanssen SA, Hasselquist D, Folstad I, Erikstad KE (2004) Costs of immunity: immune responsiveness reduced survival in a vertebrate. Proc R Soc Lond B 271:925–930
Hasselquist D, Nilsson J-Å (2012) Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim Behav 83:1303–1312
Hawley DM, Sydenstricker KV, Kollias GV, Dhondt AA (2005) Genetic diversity predicts pathogen resistance and cell-mediated immunocompetence in house finches. Biol Lett 1:326–329
Hinde CA (2006) Negotiation over offspring care? – a positive response to partner-provisioning rate in great tits. Behav Ecol 17:6–12
Hõrak P, Zilmer M, Saks L, Ots I, Karu U, Zilmer K (2006) Antioxidant protection, carotenoids and the costs of immune challenge in greenfinches. J Exp Biol 209:4329–4338
Kennedy MW, Nager RG (2006) The perils and prospects of using phytohaemagglutinin in evolutionary ecology. Trends Ecol Evol 21:653–655
Kerr AM, Gershman SN, Sakaluk SK (2010) Experimentally induced spermatophore production and immune responses reveal a trade-off in crickets. Behav Ecol 21:647–654
Klasing KC, Laurin DE, Peng RK, Fry D (1987) Immunologically mediated growth depression in chicks: influence of feed intake, corticosterone and interleukin-1. J Nutr 117:1629–1637
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Lienart GDH, Mitchell MD, Ferrari MCO, McCormick MI (2014) Temperature and food availability affect risk assessment in an ecotherm. Anim Behav 89:199–204
Lifjeld JT, Dunn PO, Whittingham LA (2002) Short-term fluctuations in cellular immunity of tree swallows feeding nestlings. Oecologia 130:185–190
Lozano GA, Ydenberg RC (2002) Transgenerational effects of maternal immune challenge in tree swallows (Tachycineta bicolor). Can J Zool 80:918–925
Martin J, de Neve L, Polo V, Fargallo JA, Soler M (2006) Health-dependent vulnerability to predation affects escape responses of unguarded chinstrap penguin chicks. Behav Ecol Sociobiol 60:778–784
Mashaly M, Hendricks G, Kalama M, Gehad A, Abbas A, Patterson P (2004) Effects of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 83:889–894
Mathot KJ, Abbey-Lee RN, Kempenaers B, Dingemanse NJ (2016) Do great tits (Parus major) suppress basal metabolic rate in response to increased perceived predation danger? A field experiment. Physiol Behav 164:400–406
McNamara KB, Wedell N, Simmons LW (2013) Experimental evolution reveals trade-offs between mating and immunity. Biol Lett 9:20130262
Møller AP, Birkhead TR (1993) Certainty of paternity covaries with paternal care in birds. Behav Ecol Sociobiol 33:261–268
Moreno J, Sanz JJ, Arriero E (1999) Reproductive effort and T-lymphocyte cell-mediated immunocompetence in female pied flycatchers. Proc R Soc Lond B 266:1105–1109
Nager RG, Wiersma P (1996) Physiological adjustment to heat in Blue Tit Parus caeruleus nestlings from a Mediterranean habitat. Ardea 84:115–125
Ots I, Kerimov B, Ivankina EV, Ilyina TA, Horak P (2001) Immune challenge affects basal metabolic rate in wintering Great Tits. Proc R Soc Lond B 268:1175–1181
Peters A, Magdeburg S, Delhey K (2011) The carotenoid conundrum: improved nutrition boosts plasma carotenoid levels but not immune benefits of carotenoid supplementation. Oecologia 166:35–43
Råberg L, Nilsson JA, Ilmonen P, Stjemman M, Hasselquist D (2000) The cost of an immune response: vaccination reduces parental effort. Ecol Lett 3:382–386
Ramírez F, Hobson KA, Wangensteen OS, Genovart M, Viscor G, Sanpera C, Jover L (2010) A physical marker for quantifying differential reproductive investment between the sexes in Yellow-legged gulls (Larus michahellis). J Exp Mar Biol Ecol 396:48–52
Rehling A, Spiller I, Krause ET, Nager RG, Monaghan P, Trillmich F (2012) Flexibility in the duration of parental care: zebra finch parents respond to offspring needs. Anim Behav 83:35–39
Reinertsen RE (1983) Nocturnal hypothermia and its energetic significance for small birds living in the Arctic and subarctic regions. A review. Polar Res 1(3):269–284
Ros AFH, Groothuis TGG, Apanius V (1997) The relation among gonadal steroids, immunocompetence, body mass, and behaviour in young black-headed gulls. Am Nat 150:201–219
Ros AFH, Correia M, Wigfield JC, Oliveira RF (2008) Mounting an immune response correlates with decreased androgen levels in male peafowl Pavo cristatus. J Ethol. https://doi.org/10.1007/s10164-008-0105-0
Roth O, Kurtz J, Reusch TBH (2010) A summer heat wave decreases the immunocompetence of the mesograzer, Idotea baltica. Mar Biol 157:1605–1611
Saino N, Calza S, Møller AP (1997) Immunocompetence of nestling barn swallows in relation to brood size and parental effort. J Anim Ecol 66:827–836
Scholander PF, Hock R, Walters V, Johnson F, Irving L (1950) Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99:237–258
Seppälä O, Jokela J (2011) Immune defence under extreme ambient temperature. Biol Lett 7:119–122
Smiths JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Snoeijs T, Eens M, Van Den Steen E, Pinxten R (2007) Kinetics of primary antibody responses to sheep red blood cells in birds: a literature review and new data from great tits and European starlings. Anim Biol 57:79–95
Soler JJ, Moreno J, Potti J (2003) Environmental, genetic and maternal components of immunocompetence of nestling pied flycatchers from a cross-fostering study. Evol Ecol Res 5:259–272
Sonerud GA, Steen R, Løw LM, Røed LT, Skar K, Selås V, Slagsvold T (2013) Size-biased allocation of prey from male to offspring via female: family conflicts, prey selection and evolution of sexual size dimorphism in raptors. Oecologia 172:93–107
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Tella JL, Scheuerlein A, Ricklefs RE (2002) Is cell-mediated immunity related to the evolution of life- history strategies in birds? Proc R Soc Lond B 269:1059–1066
Thusius KJ, Dunn PO, Peterson KA, Whittingham LA (2001) Extrapair paternity is influenced by breeding synchrony and density in the common yellowthroat. Behav Ecol 12(5):633–639
Trigo S, Mota PG (2015) What is the value of a yellow patch? Assessing the signalling role of yellow colouration in the European serin. Behav Ecol Sociobiol. https://doi.org/10.1007/s00265-014-1860-2
Tripet F, Richner H (1997) Host responses to ectoparasites: food compensation by parent blue tits. Oikos 78:557–561
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the decent of man 1871–1971. Aldine, Chicago, pp 136–179
Trust KA, Miller MW, Ringelman JK, Orme IM (1990) Effects of ingested lead on antibody production in mallards (Anas platyrhynchos). J Wildl Dis 26(3):316–322
Verhulst S, Riedstra B, Wiersma P (2005) Brood size and immunity costs in zebra finches Taeniopygia guttata. J Avian Biol 36:22–30
Vinkler M, Bainova H, Albrecht T (2010) Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct Ecol 24:1081–1086
Vinkler M, Schnitzer J, Munclinger P, Albrecht T (2012) Phytohaemagglutinin skin-swelling test in scarlet rosefinch males: low-quality birds respond more strongly. Anim Behav 83:17–23
Zając T (1999) Phenotypic selection on body size in the Great Tit Parus major (Niepołomice Forest, Poland). Acta Ornithol 34:219–226
Acknowledgements
The authors thank Subject Editor Leonida Fusani, the one anonymous reviewer and the Editor-in-Chief Franz Bairlein for many useful comments on the previous versions of the article. Rafał Martyka, Mariusz Cichoń, Rafał Bobrek, Jakub Hasny and Katarzyna Zembaczyńska kindly helped with the nest box checks. This research was partially financed by the Jagiellonian University grant DS/WBiNoZ/INoS/767/10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study complies with the current law of Poland and was carried out after obtaining permission of the local ethics committee in Kraków.
Additional information
Communicated by L. Fusani.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grzędzicka, E., Kubacka, J. Ambient temperature impacts the effect of experimental immunisation of Great Tit and Eurasian Blue Tit females on the PHA response of their offspring. J Ornithol 159, 761–770 (2018). https://doi.org/10.1007/s10336-018-1534-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-018-1534-3