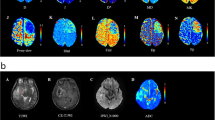
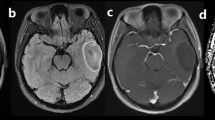
Abstract
Objective
To assess the performance of hybrid multi-dimensional magnetic resonance imaging (HM-MRI) in quantifying hematoxylin and eosin (H&E) staining results, grading and predicting isocitrate dehydrogenase (IDH) mutation status of gliomas.
Materials and methods
Included were 71 glioma patients (mean age, 50.17 ± 13.38 years; 35 men). HM-MRI images were collected at five different echo times (80–200 ms) with seven b-values (0–3000 s/mm2). A modified three-compartment model with very-slow, slow and fast diffusion components was applied to calculate HM-MRI metrics, including fractions, diffusion coefficients and T2 values of each component. Pearson correlation analysis was performed between HM-MRI derived fractions and H&E staining derived percentages. HM-MRI metrics were compared between high-grade and low-grade gliomas, and between IDH-wild and IDH-mutant gliomas. Using receiver operational characteristic (ROC) analysis, the diagnostic performance of HM-MRI in grading and genotyping was compared with mono-exponential models.
Results
HM-MRI metrics FDvery-slow and FDslow demonstrated a significant correlation with the H&E staining results (p < .05). Besides, FDvery-slow showed the highest area under ROC curve (AUC = 0.854) for grading, while Dslow showed the highest AUC (0.845) for genotyping. Furthermore, a combination of HM-MRI metrics FDvery-slow and T2Dslow improved the diagnostic performance for grading (AUC = 0.876).
Discussion
HM-MRI can aid in non-invasive diagnosis of gliomas.





Similar content being viewed by others
Data availability
For ethical considerations, the data supporting the study's conclusions are not publicly available; however, they are available from the corresponding author upon appropriate request.
Abbreviations
- CNS:
-
Central nervous system
- DWI:
-
Diffusion weighted imaging
- IDH:
-
Isocitrate dehydrogenase
- ADC:
-
Apparent diffusion coefficient
- LGG:
-
Low-grade glioma
- HGG:
-
High-grade glioma
- IDH-MUT:
-
IDH-mutant glioma
- IDH-WILD:
-
IDH-wild glioma
- HM-MRI:
-
Hybrid multi-dimensional MRI
- GRE:
-
Gradient echo
- TR:
-
Repetition time
- TE:
-
Echo time
- FOV:
-
Field of view
- FSE:
-
Fast spin echo
- FLAIR:
-
Fluid-attenuated inversion recovery
- EES:
-
Extravascular extracellular space
- BBB:
-
Brain-blood barrier
- ROI:
-
Regions of interest
- IHC:
-
Immune-histochemical
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the receiver operating characteristic curve
- LR:
-
Logistic regression
- WHO:
-
World Health Organization
- H&E:
-
Hematoxylin and eosin
- AQP4:
-
aquaporin-4
References
Burgenske DM, Yang J, Decker PA et al (2019) Molecular profiling of long-term IDH-wildtype glioblastoma survivors. Neuro Oncol. https://doi.org/10.1093/neuonc/noz129
Gritsch S, Batchelor TT, Gonzalez Castro LN (2022) Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. https://doi.org/10.1002/cncr.33918
Komori T (2022) Grading of adult diffuse gliomas according to the 2021 WHO classification of tumors of the central nervous system. Lab Invest. https://doi.org/10.1038/s41374-021-00667-6
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. https://doi.org/10.1093/neuonc/noab106
Uribe-Cardenas R, Giantini-Larsen AM, Garton A, Juthani RG, Schwartz TH (2022) Innovations in the diagnosis and surgical management of low-grade gliomas. World Neurosurg. https://doi.org/10.1016/j.wneu.2022.06.070
Szychot E, Youssef A, Ganeshan B et al (2021) Predicting outcome in childhood diffuse midline gliomas using magnetic resonance imaging based texture analysis. J Neuroradiol. https://doi.org/10.1016/j.neurad.2020.02.005
Narvaez O, Svenningsson L, Yon M, Sierra A, Topgaard D (2022) Massively multi-dimensional diffusion-relaxation correlation MRI. Front Phys. https://doi.org/10.3389/fphy.2021.793966
Jalnefjord O, Andersson M, Montelius M et al (2018) Comparison of methods for estimation of the intravoxel incoherent motion (IVIM) diffusion coefficient (D) and perfusion fraction (f). MAGMA. https://doi.org/10.1007/s10334-018-0697-5
Hu LS, Hawkins-Daarud A, Wang L, Li J, Swanson KR (2020) Imaging of intratumoral heterogeneity in high-grade glioma. Cancer Lett. https://doi.org/10.1016/j.canlet.2020.02.025
Guo D, Jiang B (2023) Noninvasively evaluating the grade and IDH mutation status of gliomas by using mono-exponential, bi-exponential diffusion-weighted imaging and three-dimensional pseudo-continuous arterial spin labeling. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2023.110721
Maynard J, Okuchi S, Wastling S et al (2020) World Health Organization grade II/III glioma molecular status: prediction by MRI morphologic features and apparent diffusion coefficient. Radiology. https://doi.org/10.1148/radiol.2020191832
Gu W, Fang S, Hou X, Ma D, Li S (2021) Exploring diagnostic performance of T2 mapping in diffuse glioma grading. Quant Imaging Med Surg. https://doi.org/10.21037/qims-20-916
Kern M, Auer TA, Picht T, Misch M, Wiener E (2020) T2 mapping of molecular subtypes of WHO grade II/III gliomas. BMC Neurol. https://doi.org/10.1186/s12883-019-1590-1
Cao M, Ding W, Han X et al (2019) Brain T1ρ mapping for grading and IDH1 gene mutation detection of gliomas: a preliminary study. J Neurooncol. https://doi.org/10.1007/s11060-018-03033-7
Springer E, Cardoso PL, Strasser B et al (2022) MR fingerprinting—a radiogenomic marker for diffuse gliomas. Cancers (Basel). https://doi.org/10.3390/cancers14030723
Auer TA, Kern M, Fehrenbach U et al (2021) T2 mapping of the peritumoral infiltration zone of glioblastoma and anaplastic astrocytoma. Neuroradiol J. https://doi.org/10.1177/1971400921989325
Bontempi P, Rozzanigo U, Amelio D et al (2021) Quantitative multicomponent T2 relaxation showed greater sensitivity than flair imaging to detect subtle alterations at the periphery of lower grade gliomas. Front Oncol. https://doi.org/10.3389/fonc.2021.651137
Toh CH, Chen YL, Hsieh TC et al (2006) Glioblastoma multiforme with diffusion-weighted magnetic resonance imaging characteristics mimicking primary brain lymphoma. case report. J Neurosurg. https://doi.org/10.3171/jns.2006.105.1.132
Kim Y, Lee SK, Kim JY, Kim JH (2023) Pitfalls of diffusion-weighted imaging: clinical utility of T2 shine-through and T2 black-out for musculoskeletal diseases. Diagnostics (Basel). https://doi.org/10.3390/diagnostics13091647
Chatterjee A, Mercado C, Bourne RM et al (2022) Validation of prostate tissue composition by using hybrid multi-dimensional MRI: correlation with histologic findings. Radiology. https://doi.org/10.1148/radiol.2021204459
Chatterjee A, Antic T, Gallan AJ et al (2022) Histological validation of prostate tissue composition measurement using hybrid multi-dimensional MRI: agreement with pathologists’ measures. Abdom Radiol (NY). https://doi.org/10.1007/s00261-021-03371-7
Lee GH, Chatterjee A, Karademir I et al (2022) Comparing radiologist performance in diagnosing clinically significant prostate cancer with multiparametric versus hybrid multi-dimensional MRI. Radiology. https://doi.org/10.1148/radiol.211895
Zeng Q, Shi F, Zhang J, Ling C, Dong F, Jiang B (2018) A modified tri-exponential model for multi-b-value diffusion-weighted imaging: a method to detect the strictly diffusion-limited compartment in brain. Front Neurosci. https://doi.org/10.3389/fnins.2018.00102
Cao M, Wang X, Liu F, Xue K, Dai Y, Zhou Y (2023) A three-component multi-b-value diffusion-weighted imaging might be a useful biomarker for detecting microstructural features in gliomas with differences in malignancy and IDH-1 mutation status. Eur Radiol. https://doi.org/10.1007/s00330-022-09212-5
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. https://doi.org/10.1016/j.neuroimage.2006.01.015
Boruah D, Deb P, Srinivas V, Mani NS (2014) Morphometric study of nuclei and microvessels in gliomas and its correlation with grades. Microvasc Res. https://doi.org/10.1016/j.mvr.2014.03.002
Zaccagna F, Riemer F, Priest AN et al (2019) Non-invasive assessment of glioma microstructure using VERDICT MRI: correlation with histology. Eur Radiol. https://doi.org/10.1007/s00330-019-6011-8
Raja R, Rosenberg GA, Caprihan A (2018) MRI measurements of blood-brain barrier function in dementia: a review of recent studies. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2017.10.034
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med. https://doi.org/10.1056/NEJMoa0808710
Furtado FS, Mercaldo ND, Vahle T et al (2023) Simultaneous multislice diffusion-weighted imaging versus standard diffusion-weighted imaging in whole-body PET/MRI. Eur Radiol. https://doi.org/10.1007/s00330-022-09275-4
Slator PJ, Palombo M, Miller KL et al (2021) Combined diffusion-relaxometry microstructure imaging: current status and future prospects. Magn Reson Med. https://doi.org/10.1002/mrm.28963
Acknowledgements
This work was partly supported by the National NSFC International (regional) Cooperation and Exchange Project (Grant No. 82111530204), partly supported by Swedish Foundation for International Cooperation in Research and Higher Education (Grant No. CH2020-8775), and partly supported by Hubei Provincial Natural Science Foundation of China (Grant No. 2021CFB099).
Author information
Authors and Affiliations
Contributions
Wenbo Sun: investigation; data curation; validation; writing—original draft; visualization; formal analysis. Dan Xu: investigation; data curation; validation; writing—original draft; visualization; formal analysis. Huan Li: data curation; project administration. Sirui Li: investigation; methodology. QingJia Bao: writing—review & editing; resources. Xiaopeng Song: software; resources. Daniel Topgaard: supervision; conceptualization; funding acquisition; writing-review & editing. Haibo Xu: supervision; conceptualization; funding acquisition; writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
All enrolled subjects provided informed consent, and the ethics committee of our hospital approved this prospective study (Ethics number 2020109).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, W., Xu, D., Li, H. et al. Quantifying H&E staining results, grading and predicting IDH mutation status of gliomas using hybrid multi-dimensional MRI. Magn Reson Mater Phy (2024). https://doi.org/10.1007/s10334-024-01154-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10334-024-01154-x