Abstract
Objective
The objective of the study was to evaluate the ability of the noninvasive magnetic resonance techniques to monitor the scaffold-aided process of articular cartilage repair.
Materials and methods
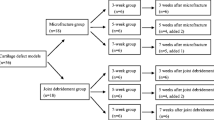
Defects of 4 mm in diameter and 3 mm in depth were created in right knees of 30 adolescent white New Zealand rabbits. Fourteen rabbits were implanted with poly(lactide-co-glycolic acid) (PLGA) scaffold trimmed to match the size and the shape of the defect (PLGA+ group). No procedure was applied to the remaining 16 animals (PLGA− group). Animals were sacrificed sequentially at 4, 12, and 24 weeks after the surgery and magnetic resonance T 2-weighted images (400 MHz) of the dissected bone plugs at eight different echo times were taken to derive T 2 relaxation time. The images and the T 2 time dependencies versus the tissue depth were statistically analyzed. Histological results of bone plugs were evaluated using semiquantitative histological scales.
Results
The results obtained for PLGA repair tissue were evaluated versus the PLGA− group and the healthy tissue harvested from the opposite knee (reference group), and compared with histological results (hematoxylin and eosin staining). The magnetic resonance images and T 2 relaxation time profiles taken 4 weeks after surgery for both the PLGA− and PLGA+ group did not reveal the tissue reconstruction. After 12 weeks of treatment T 2 time dependence indicates a slight reconstruction for PLGA+ group. The T 2 time dependence obtained for PLGA+ samples taken after 24 weeks of treatment resembled the one observed for the healthy cartilage, indicating tissue reconstruction in the form of fibrous cartilage. The tissue reconstruction was not observed for PLGA− samples.
Conclusion
The study revealed correlation between magnetic resonance and histology data, indicating the potential value of using MRI and spatial variation of T 2 as the noninvasive tools to evaluate the process of articular cartilage repair. It also suggested, that the PLGA scaffold-aided treatment could help to restore the proper architecture of collagen fibrils.
Similar content being viewed by others
Abbreviations
- PGA:
-
Poly(glycolic acid)
- PLA:
-
Poly(lactic acid)
- PLGA:
-
Poly(lactide-co-glycolic acid)
- ROI:
-
Region of interest
- FOV:
-
Field of view
- SFM:
-
Serum-free medium
- PLGA+:
-
Group treated with PLGA scaffold
- PLGA−:
-
Group treated without PLGA scaffold
References
Buckwalter JA (1997) Tissue design and chondrocyte matrix interaction. J Bone Joint Surg Am 79: 600–611
Maroudas A (1975) Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12: 233–248
Mayne R, Irwin MH (1986) Collagen types in cartilage. In: Kuettner KE, Schleyerbach R, Hascall VC(eds) Articular cartilage biochemistry. Raven Press, New York, pp 22–23
Lu L, Zhu X, Valenzuela RG, Currier BL, Yaszemski MJ (2001) Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop Relat Res 391: 251–270
Wu XS et al (1995) Synthesis and properties of biodegradable lactic/glycolic acid polymers. In: (eds) Encyclopedic handbook of biomaterials and bioengineering. Marcel Dekker, New York, pp 1015–1054
Lewis DH (1990) Controlled release of bioactive agents from lactide/glycolide polymers. In: Chasin M, Langer R(eds) Biodegradable polymers as drug delivery systems. Marcel Dekker, New York
Moran JM, Pazzano D, Bonassar LJ (2003) Characterization of polylactic acid–polyglycolic acid composites for cartilage tissue engineering. Tissue Eng 9(1): 63–70
Yoon JJ, Park TG (2001) Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res 55(3): 401–408
Tice TR, Cowsar DR (1984) Biodegradable controlled-release parenteral systems. Pharm Technol 11: 26–35
Kitchell JP, Wise DL (1985) Poly(lactic/glycolic acid) biodegradable drug-polymermatrix systems. Methods Enzymol 112: 436–448
Brady JM, Cutright DE, Miller RA, Battistone GC (1973) Resorption rate, route of elimination and ultrastructure of the implant site of polylactic acid in the abdominal wall of the rat. J Biomed Mater Res 7: 155–166
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A(Suppl 2): 58–69
Xia Y (1998) Relaxation anisotropy in cartilage by NMR microscopy (μMRI) at 14-μm resolution. Magn Reson Med 39: 941–949
Rubenstein JD, Kim JK, Morava-Protzner I, Stanchev PL, Henkelman RM (1993) Effects of collagen orientation on MR imging characteristics of bovine articular cartilage. Radiology 188: 219–226
Marcos M, Cano P, Fantazzini P, Garavaglia C, Gomez S, Garrido L (2006) NMR relaxometry and imaging of water absorbed in biodegradable polymer scaffold. Magn Reson Imaging 24: 89–95
Grunder W, Wagner M, Werner A (1998) MR-microscopic visualization of anisotropic internal cartilage structures using the magic angle technique. Magn Reson Med 39: 435–442
Xia Y (2000) Magic-angel effect in magnetic resonance imaging of articular cartilage. Invest Radiol 35: 602–621
Mlynarik V, Szomolanyi P, Toffanin R, Vittur F, Trattnig S (2004) Transverse relaxation mechanisms in articular cartilage. J Magn Reson 169: 300–307
Xia Y, Moody BJ, Alhadlaq H (2002) Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (μMRI) study. Magn Reson Med 48: 460–469
Modl JM, Sether LA, Haughton VM, Kneeland JB (1990) Articular cartilage correlation of histologic zones with signal intensity at MR imaging. Radiology 176: 853–855
Mlynarik V, Degrassi A, Toffanin R, Vittur F, Cova M, Pozzi-Mucelli RS (1996) Investigation of laminar appearance of articular cartilage by means of magnetic resonance microscopy. Magn Reson Imaging 14: 435–442
Xia Y, Moody JB, Burton-Wurster N, Lust G (2001) Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthr Cartil 9: 393–406
Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ (1994) Anisotropy of NMR properties of tissues. Magn Reson Med 32: 592–601
Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS (2001) T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 46: 487–493
Watrin-Pinzano A, Ruaud JP, Cheli Y, Gonord P, Grossin L, Gillet P, Blum A, Payan E, Olivier P, Guillot G, Netter P, Loeuille D (2004) T2 mapping: an efficient MR quantitative technique to evaluate spontaneous cartilage repair in rat patella. Osteoarthr Cartil 12: 191–200
Nieminen MT, Toyras J, Laasanen MS, Silvennoinen J, Helminen HJ, Jurvelin JS (2004) Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J Biomech 37: 321–328
Watrin-Pinzano A, Ruaud JP, Cheli Y, Gonord P, Grossin L, Bettembourg-Brault I, Gillet P, Payan E, Guillot G, Netter P, Loeuille D (2004) Evaluation of cartilage repair tissue after biomaterial implementation in rat patella by using T2 mapping. Magn Reson Mater Phys 17: 219–228
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Government Granted Project KBN 3 PO5 E 049 23.
Rights and permissions
About this article
Cite this article
Zalewski, T., Lubiatowski, P., Jaroszewski, J. et al. Scaffold-aided repair of articular cartilage studied by MRI. Magn Reson Mater Phy 21, 177–185 (2008). https://doi.org/10.1007/s10334-008-0108-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-008-0108-4