Abstract
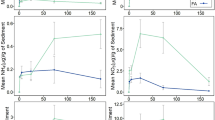
In this study, we propose a method to create a Sphagnum wetland in an urban ecosystem by collecting basic information about Sphagnum growth and decomposition. We constructed six groups of Sphagnum microcosms (1 m × 1 m) with three replicates. A factorial design with two planting methods (capitulum without stem and capitulum with stem) and three levels of nitrogen addition (0, 2, and 6 g N m−2 year−1) were prepared. Changes in length, dry mass, and decomposition rates of Sphagnum were monitored over a growing season. The effect of N concentration on production varied for the different planting methods. Production of Sphagnum increased with N concentration in the capitulum without the stem treatment (−D) than in the with stem treatment (+D). Adding N affected the decomposition rate in both with and without stem treatments. Decomposition rate increased with added nitrogen. Planting without stems (–D) was an effective design for a high production and low decomposition of Sphagnum. Net primary production was 187–260 g m−2 year−1 for dry mass and 15.5– 26.5 mm year−1 for length, whereas decomposition rates were 10.9–14.7 % mass loss per year. These values are comparable to those from natural bogs. The overall results indicate that constructing Sphagnum wetlands can be successfully employed as a greening technology in urban ecosystems, even in mid-latitudes.



Similar content being viewed by others
References
Aerts R, Wallen B, Malmer N (1992) Growth-limiting nutrients in Sphagnum-dominated bogs subject to low a high atmospheric nitrogen supply. J Ecol 80:131–140
Aldous AR (2002) Nitrogen translocation in Sphagnum mosses: effects of atmospheric nitrogen deposition. New Phytol 156:241–253
Berendse F, Van Breeman N, Rydin H, Buttler A, Heijmans M, Hoosbeek MR, Lee JA, Mitchell E, Saarinen T, Vasander H, Wallen B (2001) Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Glob Change Biol 7:591–598
Bragazza L, Tahvanainen T, Kutnar L, Rydin H, Limpens J, Hájek M, Grosvernier P, Hájek T, Hajkova P, Hansen I, Iacumin P, Gerdol R (2004) Nutritional constraints in ombrotrophic sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytol 163:609–616
Bragazza L, Rydin H, Ferdol R (2005) Multiple gradients in mire vegetation : a comparison of a Swedish and an Italian bog. Plant Ecol 177:223–236
Bragazza L, Buttler A, Habermacher J, Brancaleoni L, Gerdol R, Fritze H, Hanajik P, Laiho R, Johnson D (2012) High nitrogen deposition alters the decomposition of bog plant litter and reduces carbon accumulation. Glob Change Biol 18:1163–1172
Breemen N (1995) How Sphagnum bogs down other plants. Trends Ecol Evol 10:270–275
Breeuwer A, Monique MPDH, Maurits G, Bjorn JMR, Frank B (2009) Response of Sphagnum species mixtures to increased temperature and nitrogen availability. Plant Ecol 204:97–111
Clymo RS (1970) The growth of Sphagnum: method of measurement. J Ecol 58:13–49
Clymo RS, Tirunen J, Tolonen K (1998) Carbon accumulation in peatland. Oikos 81:368–388
Currey PM, Johnson D, Sheppard LJ (2010) Turnover of labile and recalcitrant soil carbon differ in response to nitrate and ammonium deposition in an ombrotrophic peatland. Glob Change Biol 16:2307–2321
Ferguson P, Lee JA (1983) The growth of Sphagnum species in the southern Pennines. J Bryol 12:579–586
Ferguson P, Robinson RN, Pres MC, Lee JA (1984) Element concentrations in five Sphagnum species in relation to atmospheric pollution. J Bryol 13:107–114
Freeman C, Liska G, Ostle NJ, Lock MA, Reynolds B, Hudson J (1996) Microbial activity and enzymic decomposition processes following peatland water table drawdown. Plant Soil 180:121–127
Gerdol R, Bragazza L, Brancaleoni L (2006) Microbial nitrogen cycling interacts with exogenous nitrogen supply in affecting growth of Sphagnum papillosum. Environ Exp Botany 57:1–8
Gerdol R, Petraglia A, Bragazza L, Iacumin P, Brancaleoni L (2007) Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Glob Change Biol 13:1810–1821
Gordon C, Wynn JM, Woodin SJ (2001) Impacts of increased nitrogen supply on high Arctic heath: the importance of bryophytes and phosphorus availability. New Phytol 149:461–471
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol App 1:182–195
Granath G, Wiedermann MM, Joachim S (2009) Physiological responses to nitrogen and sulphur addition and raised temperature in Sphagnum balticum. Oecologia 161:481–490
Gunnarsson U (2005) Global patterns of Sphagnum productivity. J Bryol 27:269–279
Gunnarsson U, Rydin H (2000) Nitrogen fertilization reduces Sphagnum production in bog communities. New Phytol 147:527–537
Hayward PM, Clymo RS (1982) Profiles of Water Content and Pore Size in Sphagnum and Peat, and their Relation to Peat Bog Ecology. In Proceedings of the Royal Society, Series B. Biological Sciences, London, pp.299–325
Hayward PM, Clymo RS (1983) The growth of Sphagnum: experiments on, and simulation of, some effects of light flux and water-table depth. J Ecol 71:845–863
Ingram HAP (1982) Size and shape in raised mire ecosystems: a geophysical model. Nature 297:300
Janssens I, Dieleman W, Luyssaert S (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jassey VE, Chiapusio G, Binet P, Buttler A, Laggoun F, Delarue F, Bernard N, Mitchell EA, Toussaint M, Francez A, Gilbert D (2013) Above- and belowground linkages in Sphagnum peatland: climate warming affects plant–microbial interactions. Glob Change Biol 19:811–823
Johnson LC, Damman AW (1991) Species-controlled Sphagnum decay on a South Swedish raised bog. Oikos 61:234–242
Kivimäki SK, Sheppard LJ, Leith ID, Grace J (2013) Long-term enhanced nitrogen deposition increases ecosystem respiration and carbon loss from a Sphagnum bog in the Scottish Borders. Envrion Exper Botany 90:53–61
Limpens J, Heijmans MPD (2008) Swift recovery of Sphagnum nutrient concentrations after excess supply. Oecologia 157:153–161
Luken JO (1985) Zonation of Sphagnum mosses: interactions among shoot growth, growth form, and water balance. The Bryologist 88:374–379
Malmer N, Wallen B (2005) Nitrogen and phosphorus in mire plants: variation during 50 years in relation to supply rate and vegetation type. Oikos 109:539–554
Mitch WJ, Gosselink JG (2002) Wetlands, 3rd edn. Wiley, Chichester
Pind A, Freeman C, Lock MA (1994) Enzymic degradation of phenolic materials in peatlnads—measurement of phenol oxidase activity. Plant Soil 159:227–231
Rudolph HJ, Samland J (1985) Occurrence and metabolism of sphagnum acid in the cell walls of bryophytes. Phytochemistry 24:745–749
Rydin H (1985) Effects of water level on desiccation of Sphagnum in relation to surrounding Sphagnum. Oikos 45:374–379
Rydin H (1986) Competition and niche separation in Sphagnum. Canadian J Bota 64:1817–1824
Rydin H (1993) Interspecific competition between Sphagnum mosses on a raised bog. Oikos 66:413–423
Rydin H, Clymo RS (1989) Transport of carbon and phosphorus compounds about Sphagnum. In Proceedings of the Royal Society, Series B. Biological Sciences 237, London, pp. 63–84
Thompson DK, Waddington JM (2008) Sphagnum under pressure: towards an eco-hydrological approach to examining Sphagnum productivity. Ecohydrology 1:299–308
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. The Bryologist 106:395–409
Turetsky MR, Bond Lamberty B, Euskirchen E, Talbot J, Frolking S, McGuire AD, Tuittila ES (2012) The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol 196:49–67
Van Der Heihden, Berbeek SK, Kuiper PJC (2000) Elevated CO2 and increased nitrogen deposition: effects on C and N metabolism and growth of the peat moss Sphagnum recurvum P. Beauv. Var. mucronatum (Russ.) Warnst. Glob Change Biol 6:201–212
Verhoeven JTA, Toth E (1995) Decomposition of carex and Sphagnum litter in fens: effect of litter quality and inhibition by living tissue homogenates. Soil Biol Biochem 27:271–275
Vitt DH, Wieder K, Halsey LA, Turetsky M (2003) Response of Sphagnum fuscum to nitrogen deposition: a case study of ombrogenous peatlands in Alberta, Canada. Bryologist 106:235–245
Wagner DJ, Titus JE (1984) Comparative desiccation tolerance of two Sphagnum mosses. Oecologia 62:182–187
Weltzin JF, Harth C, Bridgham SD, Poster J, Vonderharr M (2001) Production and microtopography of bog bryophytes: response to warming and water-table manipulations. Oecologia 28:557–565
Wieder PK, Lang GE (1983) Net primary production of the dominant bryophytes in a Sphagnum-dominated wetland in West Virginia. The Bryologist 86:280–286
Wiedermann MM, Nordin A, Gunnarsson U, Nilsson MB, Ericson L (2007) Global change shifts vegetation and plant–parasite interactions in a boreal mire. Ecology 88:454–464
Acknowledgments
This study was supported by the Center for Aquatic Ecosystem Restoration (CAER) of the Ecostar project from the Ministry of Environment (MOE), Republic of Korea (MOE; EW33-08-11. S. Kim was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (86457858).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S., Kim, Y., Kim, Y. et al. Effects of planting method and nitrogen addition on Sphagnum growth in microcosm wetlands. Paddy Water Environ 12 (Suppl 1), 185–192 (2014). https://doi.org/10.1007/s10333-014-0427-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-014-0427-1