Abstract
Hybridization is increasingly recognized as an important mechanism of evolutionary change in the order Primates. Here, we present the first observational data supporting natural hybridization between the critically endangered purple-faced langur (Semnopithecus vetulus philbricki) and the threatened tufted gray langur (Semnopithecus priam thersites) in Kaludiyapokuna Forest Reserve in Sri Lanka. In one case study, we observed a long-term (> 1 year) mixed-species group consisting of one adult tufted gray langur male coexisting with seven adult purple-faced females. Although copulations were not observed, two infants were conceived during the male’s tenure, and the coat color of one of these infants transitioned into that intermediate between those of the two langur species. The tufted gray langur male was also aggressive toward extra-group males of both species, as well as towards purple-faced juveniles within his group. However, we never witnessed the male exhibiting aggression towards the infants conceived during his tenure. In a second case study, a female purple-faced langur visited and sexually solicited a tufted gray langur male in a known study group of this species over the course of 2 days, in what resembled a sexual consortship. Taken together, the observed mixed-species association and attempted interspecific mating suggest that hybridization is very likely in these sympatric species. Genetic data are needed to confirm and determine the extent of hybridization in the dry zone of Sri Lanka where purple-faced langurs live in sympatry with tufted gray langurs.
Similar content being viewed by others
Introduction
Although historically viewed as rare, natural hybridization between individuals from genetically distinct lineages has been documented in over 10% of primate species (Arnold and Meyer 2006; Zinner et al. 2011). Evidence for hybridization has mostly come from observations of mixed-species associations, sexual behavior between phenotypically distinct individuals, and reports of intermediate morphotypes (e.g., in pelage color) in hybrid zones where closely related species live in sympatry (e.g., Dunbar and Dunbar 1974; Phillips-Conroy et al. 1991; Aguiar et al. 2008). More recently, genetic analyses have confirmed greater levels of heterozygosity in purported hybrid zones relative to areas where species are reproductively isolated (Cortés-Ortiz et al. 2007; Pastorini et al. 2009; Charpentier et al. 2012; Matsudaira et al. 2013; Malukiewicz et al. 2015). Molecular studies have also revealed ample evidence for ancient hybridization events across the primate lineage (Zinner et al. 2009; Roberts et al. 2010; Roos et al. 2011), including in recent hominin evolution (Ackermann et al. 2019), suggesting that many lineages have experienced gene flow throughout their evolutionary history. These studies underscore the emerging view that hybridization has played a key role in the evolution and extinction of lineages within the order Primates (Ackermann et al. 2019; Cortés-Ortiz et al. 2019).
Asian colobines have emerged as one of several primate taxa whose evolutionary history has been heavily impacted by hybridization. Historical hybridization between colobine species has now been supported by a number of molecular studies (Roos et al. 2011, 2019; Wang et al. 2015; Arekar et al. 2019). Furthermore, the presence of interspecific groups, hybrid morphotypes, and hybrid-like vocalizations suggest active hybrid zones in South India (Oates 1982; Hohmann and Herzog 1985; Hohmann 1989), where the tufted gray langur (Semnopithecus priam) and the Nilgiri langur (Semnopithecus johnii) are sympatric.
Tufted gray langurs (Semnopithecus priam) are closely related to Nilgiri langurs (Semnopithecus johnii) and purple-faced langurs (Semnopithecus vetulus), and exist sympatrically with Nilgiri langurs in Southern India, and purple-faced langurs in the north central dry zone of Sri Lanka (Wang et al. 2012; Rudran et al. 2020). Despite close genetic affiliations, striking differences in morphology and pelage pattern exist between all three species. While the morphology of the tufted gray langur strongly resembles that of other gray (Hanuman) langurs from continental Asia, the purple-faced and Nilgiri langurs cluster together in phylogenetic analyses, and resemble members of the genus Trachypithecus—so much so, that they were formerly classified within Trachypithecus (Groves 2001) prior to detailed molecular analyses (Karanth et al. 2008; Osterholz et al. 2008). S. priam is also more ubiquitous, occupying both forested and urban habitats, while the other two langur species are largely confined to forested areas. In Sri Lanka, these differences are starkly apparent, with the purple-faced langur, and not the tufted gray langur, classified as critically endangered, largely because the existence of the former (and its ability to disperse and find mates) is limited by deforestation and forest fragmentation (Schwitzer et al. 2017). This pattern is even apparent in the western and southern purple-faced langur subspecies, which are found in urban and rural areas, but isolated within gardens, where trees are still present (Dela 2012).
Historical reports from Sri Lanka suggest that tufted gray and purple-faced langurs lived in sympatry long before large-scale habitat loss led to the latter being classified as critically endangered (Phillips 1935). Moreover, recent reports have confirmed that the two species continue to coexist in numerous locations in the Sri Lankan dry zone (Ripley 1965; Vandercone 2011; Kumara et al. 2019). However, to date, there have been no reports of hybridization between them. Here, we describe two observational case studies that support potential hybridization between tufted gray (Semnopithecus priam thersites) and purple-faced langurs (Semnopithecus vetulus philbricki) in the north central dry zone of Sri Lanka.
Methods
Study site and study subjects
Demographic and ad libitum behavioral data were collected on one purple-faced and one tufted gray langur group at Kaludiyapokuna Forest Reserve (KFR) in the north central dry zone of Sri Lanka. KFR is a 13-km2 forest fragment located in the Matale District (7°52.5′N, 80°44.1′E) of Sri Lanka. KFR overlaps with an archaeological site, but contains an intact forest community with several species of large mammals, including toque macaques (Macaca sinica), leopards (Panthera pardus) and Asian elephants (Elephas maximus). Annual rainfall at KFR averages ~ 1250 mm, most of which falls during the northeastern monsoon from December to February and convectional rains from October to November.
Previous research (Vandercone 2011) in KFR found that purple-faced langurs were mostly characterized by a one-male/multi-female group composition (six of six groups), while tufted gray langurs were more often multi-male–multi-female (three of three groups). Furthermore, as reported for some other sites (Ripley 1965; Kumara et al. 2019), tufted gray and purple-faced langur groups had overlapping home ranges, despite territoriality between groups of the same species. Interspecies encounters were common (observed during 75% of study months), with rates peaking to once per day in March. The majority of these encounters were relatively peaceful; however, purple-faced langurs were routinely displaced, resulting in reduced feeding time and altered substrate use when tufted gray langurs were present.
The current study focuses on events observed in one purple-faced group and one tufted gray langur group followed from September 2018 to March 2020 in KFR. The data were primarily derived from observations made from September 2018 to January 2020, prior to reductions in manpower and restrictions due to the coronavirus disease pandemic. Although detailed accounts of intergroup encounters were not the focus of data collection during this time period, we can confirm that the pattern of species-specific group compositions, territorial overlap between species, and purple-faced langur displacement following interspecies encounters were broadly similar to what has been previously reported.
We followed the purple-faced langur group (C group; formerly the PF0 group) for 9.8 ± 4.7 days (range = 0–17 days) per month, from September 2018 to January 2020, first as part of a habituation process (September 2018–March 2019), and then as part of routine demographic and behavioral data collection for individually recognized, habituated individuals (April 2019–January 2020). The tufted gray langur group was followed for 10.8 ± 5.4 days (range = 1–20 days) per month, beginning in July 2018. As of January 2019, all adult tufted gray langur males (n = 5) were habituated and could be recognized at an individual level. By contrast, adult females were still wary of observers in January 2020, with only six of seven of them individually recognizable by the field team.
Results
Case study 1: tufted gray langur male immigrates into purple-faced langur group
From 20 September to 20 December 2018, we observed one purple-faced adult male, Charlie, and one tufted gray langur adult male, Crazy, intermittently present in C group. Because we were in the process of habituating the langurs, we were unsure whether Crazy was a new immigrant at this time, but field assistants for a pilot study conducted from January to March 2018 had identified only one adult male—a purple-faced individual—in C group. This suggests that the tufted gray langur male, Crazy, had immigrated into the group sometime between April and September 2018.
Both Charlie and Crazy were present until 20 December 2018, after which only Crazy remained. Because we did not witness a fight or any injuries on either animal, we suspect that Charlie emigrated. Crazy then became the sole adult male (tufted gray langur) residing in a group of seven adult female purple-faced langurs and their offspring. He was present on 100% of contact days (n = 141 days) between 14 January 2019 and 31 January 2020.
During this period, Crazy engaged in several aggressive encounters with extra-group purple-faced and tufted gray langur males. On 13 January 2019 and 12 July 2019, C group was first chased by a group of six tufted gray langur bachelors and then by an unknown group of tufted gray langurs comprising males and females. On 25 July 2019, Crazy engaged in three teeth-grinding displays directed at male tufted gray langurs in a known group of this species (S group). On 29 August 2019, C group was chased by an unknown purple-faced langur group. On 24 September 2019, two lone purple-faced langur males chased C group and showed aggressive teeth-grinding displays towards Crazy. On 9 December 2019, Crazy was attacked by a lone purple-faced male, and on 12 January 2020, Crazy chased a lone purple-faced male. These interactions highlight the interspecific nature of male-male competition in which Crazy was involved, and are distinct from the more peaceful interspecific encounters that are generally observed between purple-faced and tufted gray langur groups in the study area.
Although no copulations or attempted copulations were recorded between Crazy and the purple-faced females in his group, two infants were born in the subsequent year, one on 24 July 2019 (Ceba, born to Chaaru), and another on 2 September 2019 (Cola, born to Cheena). As of 15 January 2020, both infants had shed their light brownish-red natal coats. The older infant (27 weeks), Ceba, had developed the darker gray-brown coat characteristic of adult purple-faced langurs. By contrast, the younger infant, Cola (18 weeks), had developed strikingly lighter pelage on the crown of the head and body compared to Ceba and the other purple-faced langurs—a coloration that persisted for at least 2 months following the main study period (in March 2020, when we temporarily stopped following groups due to the coronavirus disease pandemic). Cola’s pelage color appeared to be intermediate between the darker brown of purple-faced langur coats and the lighter beige-gray of tufted gray langur coats (Fig. 1). Cola also possessed other features reminiscent of tufted gray langurs, including forward protruding eyebrows, and a hair tuft that met in the midline of the crown of the head, features that are commonly found in adult tufted gray langurs, but not in purple-faced langurs. These features were not present in the other infant, Ceba, which had a more rounded crown, and non-protruding eyebrows. The combination of light coloration, pointed hair tuft, and protruding eyebrows, made Cola stand out from the other purple-faced langurs that have been observed in this population, including other infants.
Notably, neonatal coat color in the purple-faced langur population at KFR and elsewhere is quite variable (Vandercone, unpublished data; Dela, personal communication), ranging from reddish brown to brownish-gray, but is typically lighter than the adult coat color. Previous studies indicate that the transition in coat color to that of adults typically occurs by 12–16 weeks of age for purple-faced langurs (Rudran 1973). By contrast, neonatal gray langurs typically have a black coat that fades to beige-gray by 12–20 weeks of age (Jay 1965). The transition from reddish brown to an apparently lighter beige-gray documented for Cola’s coat was thus unique. Based on a projected 200-day gestation length of comparable-sized Asian colobines (Borries et al. 2011), Ceba would have been conceived around 5 January 2019 and Cola around 14 February 2019, which places the conception of Cola squarely during Crazy’s tenure as the sole adult male in the group.
Although Crazy’s interactions with juveniles and infants born the previous year were always aggressive, we witnessed no aggression between Crazy and the new infants. In addition, Crazy groomed and sat within 1 m of the mothers while they were holding these infants. As of 30 March 2020, both infants were still alive.
Case study 2: two-day visit of a female purple-faced langur to a tufted gray langur group
During a 2-day period, 6 and 7 January 2020, an unknown female purple-faced langur visited a known tufted gray langur group, S group. At the time of the visit, S group comprised five adult males and seven adult females. On the morning of 6 January 2020 (9:58 a.m.), the purple-faced female presented to one of the tufted gray langur adult males, Sandy. We observed that the female had a swollen vulva. Sandy visually inspected and sniffed the genitalia of the female. Six minutes later (11:04 a.m.), the purple-faced female engaged in head-shaking behavior, an extreme form of colobine sexual solicitation, while also presenting to Sandy. Sandy then mounted her. For the rest of the morning/afternoon, the purple-faced female remained next to Sandy. She presented another two times, engaged in headshaking behavior (a proceptive colobine behavior) on one of these occasions, and was again mounted by Sandy. The pair appeared to be in consort for most of the day. On one occasion, the pair was harassed by a tufted gray langur female, who chased them. The next day, on 7 January 2020, the same purple-faced female was observed to again solicit (headshake and present) Sandy, who again mounted her, although the pair were not observed to copulate. In the afternoon, we no longer saw the purple-faced female. At 15:32 p.m., Sandy mounted and copulated with Subaru, a tufted gray langur female of S group. We are unsure whether this was the same female that had harassed Sandy when he was with the unknown female purple-faced langur the day before.
Discussion
Here we present the first observational data of mixed-species groups, one infant of intermediate pelage coloration, and attempted mating between sympatric tufted gray and purple-faced langurs in KFR, in Sri Lanka. Similar observations of mixed-species or mixed-pelage groups have been reported for gibbons (Asensio et al. 2017), cercopithecines {e.g., across nearly all species of baboons (Phillips-Conroy et al. 1991; Jolly 1993; Jolly et al. 2011; Charpentier et al. 2012); Theropithecus gelada × Papio anubis (Dunbar and Dunbar 1974); guenons and vervets [e.g., Cercopithecus spp. (Detwiler 2019), Chlorocebus spp. (Mekkonnen et al. 2018)]; colobines [e.g., Semnopithecus entellus × Semnopithecus johnii (Oates 1982; Hohmann and Herzog 1985; Hohmann 1989)]}, and various New World primates [e.g., howler monkey species (Cortés-Ortiz et al. 2015); callitrichids (Malukiewicz 2019)]. In nearly all cases, hybridization has been inferred based on putative hybrid offspring with intermediate coat color or observed interspecific sexual behavior. In several cases, genetic data have also confirmed the hybrid status of individuals (Zinner et al. 2011; Ackermann et al. 2019).
Although we did not observe successful copulation between tufted gray and purple-faced langurs, the sexual solicitation of a tufted gray langur male by a purple-faced female, coupled with the immigration and continued tenure of a single tufted gray langur male in a group of purple-faced females, strongly supports the hypothesis that hybridization occurs between these species. The birth of an infant of intermediate coat color in the mixed-species group, and the lack of overt aggression directed by the tufted gray langur towards that infant, further support this hypothesis. Indeed, aggression by males towards unrelated infants is typical in gray langur species and has been attributed to sexually selected infanticide (Hrdy 1974; Borries 1997).
Notably, pelage color of both adult and neonate purple-faced langurs is quite variable in KFR and elsewhere (Dela, personal communication). Although the coats of purple-faced langurs tend to transition from lighter neonatal coats to darker adult coats (and gray langur coats from darker neonatal to lighter adult coats), our study demonstrates that this may not always be the case. Variation in coat color and the pattern of neonatal-to-adult coat color transition may be associated with ancient or ongoing hybridization between the two species.
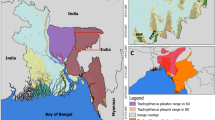
Follow-up genetic studies on the langurs in Kaludiyapokuna, as well as in other areas where tufted gray and purple-faced langurs are sympatric, are necessary to determine the possible hybrid status of some individuals and the extent of introgression into parental species across their geographic range (Fig. 2). An important part of this effort should include comparisons of the two species in question where they are sympatric, and allopatric. Currently, the Sri Lankan purple-faced langur is classified into four subspecies, including the southern (Semnopithecus vetulus vetulus), western (Semnopithecus vetulus nestor), northern (Semnopithecus vetulus philbricki), and highland (Semnopithecus vetulus monticola) groups. Of these subspecies, only the range of the northern purple-faced langur overlaps with that of S. priam, and it does so across the entirety of its range. Outside of this range of overlap S. priam exists in isolation in the eastern part of the island, its range extending as far north as Jaffna, and as far south as Yala National Park. Although the phylogenetic systematics of the purple-faced langur subspecies have not been evaluated using genetic data, it is highly possible that the northern subtype has greater signatures of hybridization with tufted gray langurs than do the other three subspecies, especially within forested environments where the northern purple-faced langur and tufted gray langur have overlapping home ranges, use the same substrates, and share a similar dietary niche (Vandercone et al. 2012).
The geographic range of the tufted gray langur (Semnopithecus priam thersites) and purple-faced langur subspecies (Semnopithecus vetulus philbricki), based on The International Union for Conservation of Nature (IUCN) Red List of Threatened Species (Rudran et al. 2020; Singh et al. 2020). Note that only the dry zone range of the purple-faced langur overlaps geographically with that of the tufted gray langur. Primate illustrations (copyright 2020 Stephen D. Nash/IUCN Species Survival Commission Primate Specialist Group) are used with permission
Genetic data will also help identify whether introgression has occurred consistently throughout the evolutionary history of these sympatric taxa, or has increased recently due to human-induced habitat loss. Hybridization can have a number of effects on evolution (Cortés-Ortiz 2019). On the one hand, introgression minimally affects parental populations if hybrids are infertile or have reduced fitness compared to parental species. However, parental species can still suffer “lost reproductive effort” because of hybrid infertility [i.e., “demographic swamping” (Wolf et al. 2001)]. Alternatively, under scenarios where hybrids have a fitness advantage, hybridization can lead to the merging of parental lineages or the formation of a new hybrid species. If introgression is unidirectional, it could even result in the gradual replacement of one parental lineage by another (i.e., “genetic swamping”).
These effects of hybridization can have clear implications for conservation, particularly for cases in which hybridization is recent, extensive, and brought about through anthropogenic changes in the environment (Todesco et al. 2016). For the langur species in Sri Lanka, sympatric coexistence clearly occurred in the dry zone long before recent habitat loss. Thus, hybridization amongst them is unlikely to be a recent phenomenon. Nevertheless, even for historically sympatric species, recent forest fragmentation and declining population size can limit mating opportunities, and thus increase the likelihood of hybridization (Detwiler et al. 2005; Zinner et al. 2011; Hamilton and Miller 2016; Todesco et al. 2016). Of the two species, the critically endangered purple-faced langur is likely more vulnerable in this scenario. As a specialized folivorous, arboreal primate, habitat fragmentation has a much greater impact on its ability to disperse and find mating opportunities. Thus molecular studies that examine the extent of both historical and more recent genetic introgression, and the details of that introgression (e.g., directionality, backcrossing, etc.), are critical to understanding the evolutionary history, future trajectory, and conservation of these taxa.
References
Ackermann RR, Arnold ML, Baiz MD et al (2019) Hybridization in human evolution: insights from other organisms. Evol Anthropol 28:189–209
Aguiar LM, Pie MR, Passos FC (2008) Wild mixed groups of howler species (Alouatta caraya and Alouatta clamitans) and new evidence for their hybridization. Primates 49:149–152
Arekar K, Parigi A, Praveen Karanth K (2019) The convoluted evolutionary history of the capped-golden langur lineage (Cercopithecidae: Colobinae)—concatenation versus coalescent analyses. bioRxiv 508929. https://doi.org/10.1101/508929
Arnold ML, Meyer A (2006) Natural hybridization in primates: one evolutionary mechanism. Zoology 109:261–276
Asensio N, José-Domínguez JM, Kongrit C, Brockelman WY (2017) The ecology of white-handed and pileated gibbons in a zone of overlap and hybridization in Thailand. Am J Phys Anthropol 163:716–728
Borries C (1997) Infanticide in seasonally breeding multimale groups of Hanuman langurs (Presbytis entellus) in Ramnagar (South Nepal). Behav Ecol Sociobiol 41:139–150
Borries C, Lu A, Ossi-Lupo K et al (2011) Primate life histories and dietary adaptations: a comparison of Asian colobines and macaques. Am J Phys Anthropol 144:286–299
Charpentier MJE, Fontaine MC, Cherel E et al (2012) Genetic structure in a dynamic baboon hybrid zone corroborates behavioural observations in a hybrid population. Mol Ecol 21:715–731
Cortés-Ortiz L, Duda TF Jr, Canales-Espinosa D et al (2007) Hybridization in large-bodied New World primates. Genetics 176:2421–2425
Cortés-Ortiz L, Agostini I, Aguiar LM et al (2015) Hybridization in howler monkeys: current understanding and future directions. In: Kowalewski MM, Garber PA, Cortés-Ortiz L, et al. (eds) Howler monkeys: adaptive radiation, systematics, and morphology. Springer, New York, pp 107–131
Cortés-Ortiz L, Roos C, Zinner D (2019) Introduction to special issue on primate hybridization and hybrid zones. Int J Primatol 40:1–8
Dela JDS (2012) Western purple-faced langurs (Semnopithecus vetulusnestor) feed on ripe and ripening fruits in human-modified environments in Sri Lanka. Int J Primatol 33:40–72
Detwiler KM (2019) Mitochondrial DNA analyses of Cercopithecus monkeys reveal a localized hybrid origin for C. mitis doggetti in Gombe National Park, Tanzania. Int J Primatol 40:28–52
Detwiler KM, Burrell AS, Jolly CJ (2005) Conservation implications of hybridization in African cercopithecine monkeys. Int J Primatol 26(3):661–684
Dunbar RIM, Dunbar P (1974) On hybridization between Theropithecus gelada and Papio anubis in the wild. J Hum Evol 3:187–192
Groves CP (2001) Primate taxonomy. Smithsonian Institution, Washington
Hamilton JA, Miller JM (2016) Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conserv Biol 30:33–41
Hohmann G (1989) Comparative study of vocal communication in two Asian leaf monkeys, Presbytis johnii and Presbytis entellus. Folia Primatol 52:27–57
Hohmann G, Herzog M (1985) Die braunen Languren Südindiens. Z Köln Zoo 28:37–41
Hrdy SB (1974) Male-male competition and infanticide among the langurs (Presbytis entellus) of Abu, Rajasthan. Folia Primatol 22:19–58
Jay P (1965) The common langur of North India. In: deVore I (ed) Primate behavior: Field Studies of Monkeys and Apes. Holt, Rinehart, & Winston, New York, pp 197–249
Jolly CJ (1993) Species, subspecies, and baboon systematics. In: Kimbel WH, Martin LB (eds) Species, species concepts and primate evolution. Springer, Boston, pp 67–107
Jolly CJ, Burrell AS, Phillips-Conroy JE et al (2011) Kinda baboons (Papio kindae) and grayfoot chacma baboons (P. ursinus griseipes) hybridize in the Kafue river valley. Zambia Am J Primatol 73:291–303
Karanth KP, Singh L, Collura RV, Stewart C-B (2008) Molecular phylogeny and biogeography of langurs and leaf monkeys of South Asia (Primates: Colobinae). Mol Phylogenet Evol 46:683–694
Kumara HWR, Nahallage CAD, Huffman MA (2019) Study on home range size and pattern among three diurnal non-human primates in Mihintale Wildlife Sanctuary in Sri Lanka. Int J Multidiscip Studies 6:56–63
Malukiewicz J (2019) A review of experimental, natural, and anthropogenic hybridization in Callithrix marmosets. Int J Primatol 40:72–98
Malukiewicz J, Boere V, Fuzessy LF et al (2015) Natural and anthropogenic hybridization in two species of Eastern Brazilian marmosets (Callithrix jacchus and C. penicillata). PLOS ONE 10:e0127268
Matsudaira K, Reichard UH, Malaivijitnond S, Ishida T (2013) Molecular evidence for the introgression between Hylobates lar and H. pileatus in the wild. Primates 54:33–37
Mekonnen A, Rueness EK, Stenseth NC, Fashing PJ, Bekele A, Hernandez-Aguilar RA, Missbach R, Haus T, Zinner D, Roos C (2018) Population genetic structure and evolutionary history of Bale monkeys (Chlorocebus djamdjamensis) in the southern Ethiopian Highlands. BMC EVOL BIOL 18:106–106
Oates JF (1982) Coat color aberrations in Presbytis johnii: a founder effect? Primates 23:307–311
Osterholz M, Walter L, Roos C (2008) Phylogenetic position of the langur genera Semnopithecus and Trachypithecus among Asian colobines, and genus affiliations of their species groups. BMC Evol Biol 8:58
Pastorini J, Zaramody A, Curtis DJ et al (2009) Genetic analysis of hybridization and introgression between wild mongoose and brown lemurs. BMC Evol Biol 9:32
Phillips WWA (1935) Manual of the mammals of Ceylon. Dulan, London
Phillips-Conroy JE, Jolly CJ, Brett FL (1991) Characteristics of hamadryas-like male baboons living in anubis baboon troops in the Awash hybrid zone, Ethiopia. Am J Phys Anthropol 86:353–368
Ripley S (1965) The ecology and social behavior of the Ceylon gray langur. Unpublished doctoral thesis, University of California, Berkeley
Roberts TE, Davenport TRB, Hildebrandt KBP et al (2010) The biogeography of introgression in the critically endangered African monkey Rungwecebus kipunji. Biol Lett 6:233–237
Roos C, Zinner D, Kubatko LS et al (2011) Nuclear versus mitochondrial DNA: evidence for hybridization in colobine monkeys. BMC Evol Biol 11:77
Roos C, Liedigk R, Thinh VN et al (2019) The hybrid origin of the Indochinese gray langur Trachypithecus crepusculus. Int J Primatol 40:9–27
Rudran R (1973) The reproductive cycles of two subspecies of purple-faced langurs (Presbytis senex) with relation to environmental factors. Folia Primatol 19:41–60
Rudran, R, Dittus, W, Gamage SN, Nekaris KAI (2020) Semnopithecus vetulus. The IUCN Red List of Threatened Species 2020: e.T22042A17959452. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T22042A17959452.en
Schwitzer C, Mittermeier RA, Rylands AB et al (2017) Primates in peril: the world’s 25 most endangered primates 2016–2018. IUCN SSC Primate Specialist Group, International Primatological Society, Conservation International, and Bristol Zoological Society, Arlington
Singh M, Kumar A, Kumara HN, Nag C (2020) Semnopithecus priam ssp. priam. The IUCN Red List of Threatened Species 2020: e.T39839A17982861. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T39839A17982861.en
Todesco M, Pascual MA, Owens GL et al (2016) Hybridization and extinction. Evol Appl 9:892–908
Vandercone R (2011) Dietary shifts, niche relationships and interspecific competition in sympatric grey langur (Semnopithecus entellus) and purple-faced langur (Trachypithecus vetulus) in Sri Lanka. Doctoral thesis, Washington University. All Theses and Dissertations (ETDs). 654. https://openscholarship.wustl.edu/etd/654
Vandercone RP, Dinadh C, Wijethunga G et al (2012) Dietary diversity and food selection in Hanuman langurs (Semnopithecus entellus) and purple-faced langurs (Trachypithecus vetulus) in the Kaludiyapokuna Forest Reserve in the dry zone of Sri Lanka. Int J Primatol 33:1382–1405
Wang XP, Yu L, Roos C et al (2012) Phylogenetic relationships among the colobine monkeys revisited: new insights from analyses of complete mt genomes and 44 nuclear non-coding markers. PLOS ONE 7:e36274
Wang B, Zhou X, Shi F et al (2015) Full-length Numt analysis provides evidence for hybridization between the Asian colobine genera Trachypithecus and Semnopithecus. Am J Primatol 77:901–910
Wolf DE, Takebayashi N, Rieseberg LH (2001) Predicting the risk of extinction through hybridization. Conserv Biol 15:1039–1053
Zinner D, Groeneveld LF, Keller C, Roos C (2009) Mitochondrial phylogeography of baboons (Papio spp.)–indication for introgressive hybridization? BMC Evol Biol 9:83
Zinner D, Arnold ML, Roos C (2011) The strange blood: natural hybridization in primates. Evol Anthropol 20:96–103
Acknowledgments
We thank the two anonymous reviewers and Dr. Noah Snyder-Mackler for input on earlier drafts of this manuscript. We thank the Department of Wildlife Conservation in Sri Lanka for permission to conduct research at KFR. We also thank Stony Brook University and the University of Georgia for funding to conduct this research. All research was observational, and approved by the Institutional Animal Care and Use Committee of Stony Brook University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Lu, A., Sirimanna, D.G.R., Wijayathunga, L. et al. Mixed-species associations and attempted mating suggest hybridization between purple-faced and tufted gray langurs of Sri Lanka. Primates 62, 11–17 (2021). https://doi.org/10.1007/s10329-020-00852-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-020-00852-z