Abstract
Wheat blast is an important disease that affects cross-border food security. The causal pathogen, Pyricularia oryzae (syn. Magnaporthe oryzae) Triticum pathotype (MoT), forms a phylogenetic group distinct from the other P. oryzae pathotypes pathogenic on various gramineous plants. Here we developed MoT-specific primer sets for qPCR and LAMP detection methods, and their amplification specificity and efficiency were tested in comparison with previous reported MoT-specific primers. The amplification efficiency and specificity were affected by the polymerase premix. To evaluate the detection specificity, purified DNA samples of 15 MoT isolates, 89 non-MoT isolates of Pyricularia spp., and crude DNA from wheat plants inoculated with MoT were used. We conclude that the Sp3 primer sets for qPCR and the MoT3L primer sets for LAMP were the best in this research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyricularia oryzae (syn. Magnaporthe oryzae) is a pathogen that causes blast disease in various monocots. The P. oryzae population comprises various pathotypes with different host plant ranges, including the Oryza, Setaria, Lolium, and Eleusine pathotypes (Kato et al. 2000). These pathotype-genus specificities are attributed to the co-evolution of host plants and pathogens (Kato et al. 2000). The P. oryzae Triticum pathotype (MoT) emerged in Brazil due to a “host jump” from ryegrass likely in 1980s (Inoue et al. 2017; Urashima et al. 1993). MoT populations have spread to South America, Bangladesh, and Zambia by seed transmission (Singh et al. 2021), and become a serious threat to food security worldwide. Thus, the development of rapid and simple detection methods from contaminated seed is in great demand.
Wheat blast causes severe damages at the heading stage. Grain yield losses due to wheat blast were estimated to be in the range of 10–100% (Singh et al. 2021). However its infection cycle remains unclear since no disease symptoms are usually observed on wheat under natural condition except the heading stage. To control wheat blast, it is important to know the site of infection during wheat growth. Attempts to isolate MoT fungus from tissues without lesions (latent infection) have been reported using a drug resistance gene as a marker (Martinez et al. 2021). However, such tests are time-consuming and carry the risk of contamination with other fungi. The use of molecular diagnostic methods should improve the sensitivity for detecting MoT in plant tissues.
Previously, several MoT-specific primer sets were developed for quantitative real-time PCR (qPCR) and Loop-Mediated Isothermal Amplification (LAMP) methods. For examples, the MoT3 primer set (hereafter referred to as MoT3Q) by Pieck et al. (2017) and the C45 primer set by Thierry et al. (2020) for qPCR detection, and the MoT3 primer set (hereafter referred to as MoT3L) by Yasuhara-Bell et al. (2018) and the Group5 primer set by Thierry et al. (2020) for LAMP detection, were reported. Thierry et al. (2020) also established a protocol for large-scale testing of MoT-contaminated seeds with pre-incubation on PDB medium. While PCR or qPCR using MoT3Q primer set is commonly used for MoT diagnosis, the LAMP method could be also useful for field testing since it allows an amplification reaction at an isothermal temperature.
Although the development of pathogen-specific primer sets has been reported for many plant diseases, in most cases there are still technical problems to be solved. Non-specific amplification may occur in plant and/or environmental DNAs, or it may not be applicable to crude DNA samples. In fact, Gupta et al. (2019) pointed out that the MoT3Q primer set gave a false positive signal with the Oryza pathotype (MoO). However, there is a counterargument that MoO isolates does not give a positive signal (Yasuhara-Bell et al. 2019). The MoT3Q primer set was designed to amplify a DNA fragment in MGG_02337, encoding short-chain dehydrogenase (Pieck et al. 2017). Another primer set, C45, distinguishes between MoT and MoO by a single nucleotide polymorphism at the 3′-end of the primer (Thierry et al. 2020). Thus, the PCR conditions for specific detection of MoT using these primer sets were likely limited.
In this study, we have developed novel primer sets for the specific detection of MoT by the qPCR and LAMP assays using the telomere-to-telomere genomic sequence of the MoT isolate Br48 (Kobayashi et al. 2023). The novel primer sets were compared with the previously reported ones in terms of specificity and sensitivity based on amplification tests using 97 isolates of Pyricularia spp. stored at Kobe University (including 8 isolates of MoT from Brazil) and seven field isolates of MoT collected in Bangladesh. These strains comprise a wide range of Pyricularia fungi, encompassing an ancestral MoT population in Brazil and a newly emerging population in Bangladesh (Inoue et al. 2017; Latorre et al. 2023). In addition, we assessed crude DNA samples extracted from MoT-inoculated wheat plants as templates for qPCR and LAMP analysis in this study.
Materials and methods
Primer design
MoT-specific genomic regions were selected by mapping short reads of eight Pyricularia isolates, 70–15 (Oryza pathotype), GFSI1-7–2 (Setaria pathotype), an MZ5-1–6 (Eleusine pathotype), TP1 (Lolium pathotype), TP2 (Lolium pathotype), Br58 (Avena pathotype), Br116.5 (Triticum pathotype, MAT1-2), and Br118 (Triticum pathotype, MAT1-2) in public database to the telomere-to-telomere genomic sequence of the Br48 (Triticum pathotype, MAT1-1) (Kobayashi et al. 2023) using CLC Genomics Workbench ver. 11.0.1 with parameter settings, length fraction = 0.9 and similarity fraction = 0.9. Primers were designed using Primer3 (https://primer3.org/) for qPCR and Primer Explorer V5 (https://primerexplorer.jp/lampv5/index.html) (Eiken Chemical Co., Ltd., Tokyo, Japan) for LAMP.
Fungal isolate.
The isolates used in this study (Table 1) were maintained in a barley seed medium (Hayashi and Kato 1988) at 4 °C in the Laboratory of Plant Pathology, Kobe University. They were transferred to potato dextrose agar slants at 25 °C. One-week-old cultures were used in the experiments.
Inoculation assay
Wheat seeds (Triticum aestivum cv. Norin4) were placed on water-soaked filter paper and left overnight for germination. The seeds were grown in vermiculite for 7 days in a plant growth chamber at 23 °C. The artificial light and dark cycle in the plant growth chamber lasted for 12 h. The Pyricularia isolates were grown on oatmeal agar medium for 7 days (25 °C), after which the aerial hyphae were removed and placed under BLB light for 5–7 days to form spores. Sterile distilled water (SDW) was added to the spore-producing medium, and the fungal surfaces were rubbed using a spatula. The spore suspension was filtered through a Kimwipe S-200 filter (Cresia Corp., Tokyo, Japan). The plant leaves were spray-inoculated with a spore suspension (1 × 105 spores/mL supplemented with 0.01% Tween-20), and the inoculated plants were incubated under humid conditions in the dark for 24 h and then moved to a plant growth chamber. Leaves and leaf sheaths were harvested from control plants (non-inoculated) and plants at 1, 3, and 5 days after inoculation.
DNA extraction
(1) DNA purification
Total DNA was extracted from mycelia as previously described (Nakayashiki et al. 1999).
(2) Extraction of crude DNA from inoculated plants
Inoculated leaves and leaf sheaths were cut into 1.5 cm pieces and each piece was collected in 1.5 mL microtube. DNA extraction buffer solution A (100 µL) (Kaneka Easy DNA Extraction Kit Version 2, Kaneka Corp., Takasago, Japan) was added to the microtubes and homogenized using BioMasher II (Nippi, Tokyo, Japan). The samples were heated at 98 °C for 8 min, cooled to 25 °C, and 14 µL of the DNA extraction buffer solution B (Kaneka) was added. The samples were centrifuged at 9800 × g for 10 min, and the supernatants were transferred to new microtubes.
PCR
DNA amplification was performed using the Quick Taq HS DyeMix (Toyobo, Osaka, Japan) and a T100™ Thermal Cycler (Bio-Rad, CA, USA). The 20-μL reaction mixture containing 1 μL of template DNA, 10 μL of 2 × Quick Taq HS DyeMix, 0.2 μM of each primer, and distilled water was subjected to the following PCR conditions: 94 °C for 2 min followed by 30 cycles of 94 °C for 30 s, 53–60 °C for 30 s, and 68 °C for 30 s. PCR products were separated by electrophoresis on a 1.5% agarose gel in TAE buffer.
qPCR
DNA amplification was performed with a Thermal Cycler Dice Real Time System Single (TaKaRa Bio, Ohtsu, Japan) using Thunderbird SYBR qPCR Mix (Toyobo), KOD SYBR qPCR Mix (Toyobo), or TB Green Premix Ex Taq™ II (Takara). The 20-μL reaction mixture containing 1 μL of template DNA, 10 μL of each 2 × polymerase Mix, 0.2 μM of each primer and distilled water was subjected to the following PCR conditions: (for Thunderbird SYBR qPCR Mix and TB Green Premix Ex Taq™ II) 95 °C for 30 s, followed by 45 cycles of 95 °C for 5 s, 60 °C for 30 s, 72 °C for 30 s, (for KOD SYBR qPCR Mix) 98 °C for 2 min, followed by 45 cycles of 98 °C for 10 s, 60 °C for 10 s, and 68 °C for 30 s. After amplification, dissociation curve was analyzed to confirm whether the PCR product was the same as that of the target. Three replicates were performed, and the average Ct values were calculated by the crossing point method. Detection limit of each primer set was determined by diluting the template DNA and determining the DNA concentration at which amplification was observed in all six replicates (Kralik and Ricchi 2017).
LAMP
DNA amplification was performed with a Genelyzer FII (Canon Medical Systems, Ohtawara, Japan) using Isothermal Master Mix (OptiGene, West Sussex, UK). The 20-μL reaction mixture containing 1 μL of template DNA, 10 μL of each 2 × Isothermal Master Mix, 0.2 μM of each F3 and B3 primer, 2 μM of each FIP and BIP primer, and distilled water was incubated at 65 °C for 45 min. After amplification, the amplification time and melting curve were analyzed to confirm whether the product was identical to that of the target. If amplification was observed at different annealing peaks, it was recorded as non-specific (N.S.). Detection limit of each primer set was determined by diluting the template DNA and determining the DNA concentration at which amplification was observed in all six replicates.
Results
Development of novel PCR and LAMP primer sets for specific detection of the Triticum pathotype of P. oryzae.
To detect a Triticum pathotype-specific sequence, genomic short reads of eight P. oryzae isolates (see Materials and Methods) were mapped to the telomere-to-telomere genome sequence of Br48 (Triticum pathotype) (Kobayashi et al. 2023). These isolates include major P. oryzae pathotypes pathogenic on rice, finger millet, oat and wheat together with Lolium and Avena pathotypes, which are closely related to MoT. The mapping data showed that there are several regions in the Br48 genome that are unique to the Triticum pathotype. We used one such region in chromosome 3 to design primers for qPCR (Sp1, Sp2, and Sp3) and LAMP (Set1 to Set8) (Table S1). With the newly designed primers, amplification reactions were carried out using genomic DNA of Br48 (MoT) and Guy11 (MoO) as templates. We also tested crude DNA extracted from wheat leaves inoculated with MoT.
For qPCR, the Sp1, Sp2, and Sp3 primer sets were MoT-specific and showed higher amplification efficiencies than the previously-reported primer set, MoT3Q (Fig. 1a). Both Sp2 and Sp3 primer sets showed the most efficient amplification rate. However, since non-specific amplification from wheat genomic DNA was observed with the Sp2 primer sets (Fig. 1b), the Sp3 primer set was selected for further study.
Evaluation of the newly designed qPCR primer sets for amplification efficiency. a Amplification curves of qPCR of Guy11 or Br48 genomic DNA (10 ng/µl) with the MoT3Q, Sp1, Sp2, and Sp3 primer sets using the premix of TB Green Premix Ex Taq™ II. b Amplification (left) and dissociation (right) curves of crude DNA extracted from MoT-inoculated leaves (L) with Sp2 or Sp3 primer set. Un-inoculated (cont) plants and 3 days after inoculation (dai) plants were used
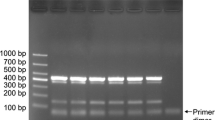
We next examined amplification specificity at different annealing temperatures with the C45, MoT3Q and Sp3 primer sets. At a low annealing temperature (53 °C), non-specific amplification from Guy11 genomic DNA was observed with the C45 and MoT3Q primers but not with the Sp3 primers (Fig. 2a), indicating that the Sp3 primer set is more resilient against false positives.
Evaluation of the previously reported PCR and qPCR primer sets for amplification efficiency. a Agarose electrophoresis images of the PCR products with MoT-specific primer sets (C45, MoT3Q, and Sp3). Genomic DNA of Guy11 (MoO) or Br48 (MoT) was used as a template. The annealing temperature of the PCR cycle ranged from 53 to 60 °C. M; molecular markers (white bars indicate 500 bp). b–d Standard curves of Br48 genomic DNA with the C45 (green), MoT3Q (red), and Sp3 (blue) primer sets using the premixes of TB Green Premix Ex Taq.™ II (b), KOD SYBR qPCR Mix (c), and Thunderbird SYBR qPCR Mix (d). DL; detection limit (pg/µl genomic DNA of Br48)
For qPCR assay, we tested three premix regents, KOD SYBR qPCR Mix, TB Green Premix Ex Taq™ II, and Thunderbird SYBR qPCR Mix. Standard curve assay indicated that the slope of standard curve was out of an acceptable range (-3.1 to -3.6) with the combinations of KOD SYBR qPCR Mix and the C45, MoT3Q, or Sp3 primer set, and Thunderbird SYBR qPCR Mix and the C45 or MoT3Q primer set (Fig. 2b–d). The amplification efficiency by the MoT3Q primer set was lower than ones by the other primer sets (Fig. 2b–d). This may be due to the large amplification size (361 bp) compared to other primer sets (Table S1). The detection limit ranged from 1 to 10 pg/μL (Fig. 2b–d). In general, efficient amplification was observed with the Sp3 primer set but amplification with the C45 primer set was considerably affected by the premix reagent used (Fig. S1a–c). Especially, the use of KOD SYBR qPCR Mix in the amplification of DNA by the C45 primer set was inefficient. In addition, nonspecific amplification from Guy11 DNA was observed with the C45 primer set using the Thunderbird SYBR qPCR Mix (Fig. S1b).
When crude DNA extracted from wheat plants by the heating method was used as a template, combination use of the MoT3Q primer set and KOD SYBR qPCR mix resulted in non-specific amplification from non-inoculated plants (Fig. S2a; Cont-LS). Such non-specific amplification was not observed when TB Green Premix Ex Taq™ II mix was used (Fig. S2b). In conclusion, TB Green Premix Ex Taq™ II was used in further experiments.
For LAMP, the newly designed primer sets were evaluated for amplification efficiency and specificity (Fig. 3a and b). Four primer sets (Set1, Set4, Set5, and Set6) showing efficient amplification were selected and compared with MoT3L primer set. On average, the MoT3L primer set displayed the highest amplification efficiency, while the Set1 and Set5 primer sets produced slightly lower but comparable amplification levels to the MoT3L primer set (Fig. 3c). Detection limit ranged from 25 (Set 1 and Set5) to 100 (Set4) pg/μL (Fig. 3c). Consistently, better amplification was observed with the MoT3L primer set than with another MoT-specific primer set, Group5, designed by Thierry et al. (2020) (Fig. S3). Amplification efficiency from Br48 DNA by the MoT3L primer set was comparable to that from Guy11 DNA by the Pot2 primer set (Fig. S3). Pot2 is the multicopy and widespread DNA type transposable element in P. oryzae, and serves as a target for PCR and LAMP assays in any P. oryzae isolate (Harmon et al. 2003; Yasuhara-Bell et al. 2018). The amplification efficiency by the Pot2 primer set was lower with Br48 DNA (detection limit was 100 pg/μL) than with Guy11 DNA (detection limit was 1 pg/μL) (Fig. S3). This may be attributed to variations in the genomic copy numbers of Pot2 and/or differences in the target sequence of Pot2 between the MoO and MoT isolates.
Evaluation of the LAMP primer sets for amplification efficiency. Amplification curves of Br48 (a) or Guy11 (b) genomic DNA (10 ng/µL) with the Set1, Set2, Set3, Set4, Set5, Set6, Set7, and Set8 primer sets. c Amplification curves of Br48 genomic DNA (10 ng/µL) with the MoT3L, Set1, Set4, Set5, and Set 6 primer sets. Times for positive reaction with standard deviation were indicated in each curve from three replicates. DL; detection limit (pg/µl genomic DNA of Br48)
Validation of the novel qPCR and LAMP primers using genomic DNA from various Pyricularia isolates and crude DNA extracted from MoT-inoculated plants.
Genomic DNA (100 ng/μL) of 97 Pyricularia isolates collected from various host plants was subjected to qPCR and LAMP assays using the novel primer sets (Sp3 for qPCR, and Set1, Set5, and Set6 for LAMP) together with the previously-reported primer sets (MoT3Q and C45 for qPCR, and MoT3L and Group5 for LAMP). In addition, seven MoT strains isolated in Bangladesh was subjected to the analyses. However, since we only had small amount of their genomic DNA samples, template DNA was used without adjusting the concentration (ranged from 30 to 80 ng/μL). In the qPCR assay, a DNA sample was considered positive if its Ct value was less than 20, and one with a CT value greater than 30 was considered a non-specific signal. All the three primer sets gave positive signals in all MoT isolates tested including ones isolated in Bangladesh (Table 1) but not in the other Pyricularia isolates tested with a few exceptions described hereafter. A positive signal was detected in Br35 isolated from Brachiaria plantaginea with the Sp3 and MoT3Q primer sets but not with the C45 primer sets. In addition, the MoT3Q primer set generated a positive signal with Br37 isolated from Setaria geniculata.
Non-specific amplification was observed frequently in Pyricularia species other than P. oryzae when the MoT3Q and Sp3 primer sets, but not the C45 primer set were used. This could be attributed to the presence of a sequence weakly homologous to the targets of the MoT3Q and Sp3 primer sets in such Pyricularia spp. This non-specific amplification was not detected when genomic DNA was used at 1 ng/μL (data not shown).
When crude DNA templates extracted from MoT-inoculated plants were used, a positive signal was detected as early as one day after inoculation with the Sp3 primer set and as early as three days after inoculation with the MoT3Q set (Fig. 4, Table 2). However, amplification with the C45 primer set was unstable as it frequently produced a non-specific signal with any crude DNA sample (Table 2). These results suggest that the Sp3 primer set offers the most sensitive qPCR method for detecting MoT using crude DNA extracted from an inoculated wheat plant.
Detection of MoT from inoculated wheat plants. Amplification (top) and dissociation (bottom) curves of qPCR of crude DNA extracted from MoT-inoculated leaves (L) and leaf sheaths (LS) with Sp3 primer set and TB Green Premix Ex Taq™ II combination. Un-inoculated (cont) plants (L; red, LS; red) and 1 (L; blue, LS; light blue) and 5 (L; green, LS; purple) days after inoculation (dai) plants were used
We next examined wheat internal standard primer sets for quantitative detection of MoT on wheat leaves. The primer set for cell division control protein (CDCP) gene (Paolacci et al. 2009) yielded efficient amplification (Fig. S4a).
In the LAMP assay, five primer sets (MoT3L, Group5, Set1, Set5, and Set6) were tested. All primer sets produced positive signals in all MoT isolates tested, except for the Set1 primer set, which produced negative results in most Bangladesh isolates (Table 1). With the Pyricularia isolates other than the MoT isolates, the LAMP assay using these primer sets yielded negative results, with a few exceptions described below. First, every primer set produced a positive signal in the B. plantaginea isolate, Br35. Secondly, the MoT3L and Group5 primer sets yielded positive signals in the Br37 and NI922 isolates, respectively. Thirdly, certain Lolium perenne isolates tested positive with the Group 5 primer set. It should be noted that the melting curve analysis revealed false positive signals due to non-specific DNA amplification with all primer sets depending on Pyricularia isolates (Table 1). The frequency of non-specific amplification was the highest with the Group5 primer set and the lowest with the Set6 primer set.
In the LAMP assay using crude DNA extracted from MoT-inoculated plants, the Set1 primer set exhibited the highest sensitivity for detection, followed by the MoT3L primer set (Table 2). With the Set6 primer set, non-specific amplification was frequently observed even in non-inoculated wheat plants (Table 2). We also evaluated wheat internal standard primer sets for LAMP to enable the quantification of MoT in wheat leaves. Approximately 4.6 kb of the CDCP genomic sequence was divided into N-, Middle-, C-regions, and three primer sets were designed (Table S1). The CDCP-middle primer set showed the highest amplification efficiency (Fig. S4b), and thus, was selected for future studies.
Discussion
In the present study, we evaluated qPCR and LAMP primer sets, in comparison with previously reported primer sets, for detecting MoT using purified genomic DNA and crude DNA extracted from MoT-inoculated plants. In the PCR experiment, non-specific amplification from MoO was observed depending on the primer set and annealing temperature. The results indicated that the Sp3 primer set developed in this study is more resilient against false positives than the previously-reported primer sets, C45 and MoT3Q.
In the qPCR experiment, the amplification efficiency was affected by qPCR premix and primer set. In particular, the C45 primer set was sensitive to the type of qPCR premix used. In addition, the C45 primer set often produced false positive signals due to non-specific amplification when plant-derived crude DNA was used as a template. The novel Sp3 primer set exhibits the highest sensitivity in detecting MoT among all tested primer sets, especially when a crude DNA template was used.
In the qPCR assay of various Pyricularia isolates, the Sp3 and MoT3Q primer sets yielded positive signals in certain non-MoT isolates such as Br35 and Br37. These primer sets also produced signals with high Ct values possibly due to non-specific DNA amplification in most of the Pyricularia isolates out of P. oryzae. It is strongly suggested that the MoT population was originated from a Lolium strain of P. oryzae with introgression of a megabase-scale DNA segment from Pyricularia spp. such as B. plantaginea isolate (Inoue et al. 2017). Later, it was shown that such introgression was a result of a sextual or parasexual cross between a Lolium strain or ancient MoT strain and B. plantaginea strain (Kobayashi et al. 2023). In fact, the target sites of the Sp3 and MoT3Q primer sets were located on a chromosomal segment derived from a B. plantaginea strain. Such an introgressed sequence from a distantly related Pyricularia spp. can be a potent target for specific detection of MoT among P. oryzae strains but it should be noted that it may give a false positive when a broader range of Pyricularia spp. is used in the assay. In addition, the introgressed chromosomal segments could share a sequence homology with the genomes of Pyricularia spp., which may produce the non-specific signals with high Ct values by the Sp3 and MoT3Q primer sets in these isolates. Therefore, to specifically detect MoT including B. plantaginea strains by qPCR, it is important to set a Ct cutoff value when purified fungal genomic DNA is used as a template.
The use of crude DNA in molecular diagnosis should simplify the handling of multiple specimens, particularly in the field. The heating DNA extraction method used in this work requires minimal equipment, making it suitable for field research. Primer sets that yielded good results using a purified fungal DNA template do not always work as well with crude DNA or a mixture of plant and fungal DNA. Therefore, the primer design should be evaluated under the final testing conditions. In this study, the combination of TB Green Premix Ex Taq™ II and the Sp3 primer set was the best to detect MoT as early as 1 day after inoculation using crude DNA extracted from the inoculated plant.
The LAMP method is applicable to crudely extracted DNA and only requires a constant temperature, making it a suitable option for field research. In this study, we tested several candidate primer sets for the LAMP assay, and found significant variance in their amplification efficiency. The Set1, Set5, and Set6 primer sets were comparable to the previously reported MoT3L primer set in amplification efficiency. When crude DNA was used as a template, the Set1 primer set exhibited the highest efficiency. However, the majority of MoT isolates from Bangladesh yielded negative results when tested with the Set1 primer set. A genome surveillance study revealed that the target region of the Set1 primer set was lost after its introduction into Bangladesh (Latorre et al. 2023). Such deletion can occur especially with a lineage specific genomic region, which may not be essential. Thus, diagnosis with multiple primer sets would make results more reliable.
In conclusion, the Sp3 primer set for qPCR and the MoT3L primer set for LAMP are among the top performing primer sets in this research. Regarding the detection limit, qPCR method (Sp3 primer set) was superior to the LAMP method (MoT3L primer set) when purified DNA was used, but both methods were comparable when crude DNA was used. In addition, the internal qPCR and LAMP primer sets for the wheat CDCP gene will be useful for quantifying the MoT in wheat plants. The qPCR and LAMP assays with a simple DNA extraction method may assist in field diagnoses and localization analyses of MoT during wheat growth.
References
Gupta DP, Avila CSR, Win J, Soanes DM, Ryder LS, Croll D, Bhattacharjee P, Hossain MS, Mahmud NU, Mehebub MS, Surovy MZ, Rahman MM, Talbot NJ, Kamoun S, Islam MT (2019) Cautionary notes on use of the MoT3 diagnostic assay for Magnaporthe oryzae wheat and rice blast isolates. Phytopathology 109:504–508
Harmon PF, Dunkle LD, Latin R (2003) A rapid PCR-based method for the detection of Magnaporthe oryzae from infected perennial ryegrass (in Japanese). Plant Dis 87:1072–1076
Hayashi N, Kato H (1988) Viability and aggressiveness of Pyricularia cultures preserved by silica gel-drying grain method. Ann Rep Kanto-Tosan Plant Prot Soc 35:12–13
Hirata K, Kusaba M, Chuma I, Osue J, Nakayashiki H, Mayama S, Tosa Y (2007) Speciation in Pyricularia inferred from multilocus phylogenetic analysis. Mycol Res 111:799–808
Inoue Y, Vy TTP, Yoshida K, Asano H, Mitsuoka C, Asuke S, Anh VL, Cumagun CJR, Chuma I, Terauchi R, Kato K, Michell T, Valent B, Farman M, Tosa Y (2017) Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science 357:80–83
Kato H, Yamamoto M, Yamaguchi-Ozaki T, Kadouchi H, Iwamoto Y, Nakayashiki H, Tosa Y, Mayama S, Mori N (2000) Pathogenicity, mating ability and DNA restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. J Gen Plant Pathol 66:30–47
Kralik P, Ricchi M (2017) A basic guide to real time PCR in microbial diagnostics: definitions, parameters, and everything. Front Microbiol 8:108
Kobayashi N, Dang TA, Pham KTM, Luciano LBG, Vu BV, Izumitsu K, Shimizu M, Ikeda K, Li WH, Nakayashiki H (2023) Horizontally transferred DNA in the genome of the fungus Pyricularia oryzae is associated with repressive histone modifications. Mol Biol Evol 40:msad186
Latorre SM, Were VM, Foster AJ, Langner T, Malmgren A, Harrant A, Asuke S, Reyes-Avila S, Gupta DR, Jensen C, Ma W, Mahmud NU, Mehebub MdS, Mulenga RM, Md MAN, Paul SK, Rabby SMF, Rahat Al AM, Ryder R, Shrestha RK, Sichilma S, Soanes DM, Singh PK, Bentley AR, Saunders DGO, Tosa Y, Croll D, Lamour KH, Islam T, Tembo B, Win J, Talbot NJ, Burbano HA, Kamoun S (2023) Genomic surveillance uncovers a pandemic clonal lineage of the wheat blast fungus. PLoS Biol 21:e3002052
Martinez SI, Wegner A, Bohnert S, Schaffrath U, Perelló A (2021) Tracing seed to seedling transmission of the wheat blast pathogen Magnaporthe oryzae pathotype Triticum. Plant Pathol 70:1562–1571
Nakayashiki H, Kiyotomi K, Tosa Y, Mayama S (1999) Transposition of the retrotransposon MAGGY in heterologous species of filamentous fungi. Genetics 153:693–703
Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10:11
Pieck ML, Ruck A, Farman ML, Peterson GL, Stack JP, Valent B, Pedley KF (2017) Genomics-based marker discovery and diagnostic assay development for wheat blast. Plant Dis 101:103–109
Singh PK, Gahtyari NC, Roy C, Roy KK, He X, Tembo B, Xu K, Juliana P, Sonder K, Kabir MR, Chawade A (2021) Wheat blast: a disease spreading by intercontinental jumps and its management strategies. Front Plant Sci 12:710707
Thierry M, Chatet A, Fournier E, Tharreau D, Ioos R (2020) A PCR, qPCR, and LAMP toolkit for the detection of the wheat blast pathogen in seeds. Plants 9:277
Urashima AS, Igarashi S, Kato H (1993) Host range, mating type and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis 77:1211–1216
Yasuhara-Bell J, Pedley KF, Farman ML, Valent B, Stack JP (2018) Specific detection of the wheat blast pathogen (Magnaporthe oryzae Triticum) by loop-mediated isothermal amplification. Plant Dis 102:2550–2559
Yasuhara-Bell J, Pieck ML, Ruck A, Farman ML, Peterson GL, Stack JP, Valent B, Pedley KF et al (2019) A response to Gupta et al. (2019) regarding the MoT3 wheat blast diagnostic assay. Phytopathology 109:509–511
Acknowledgements
This research was supported by a grant, “International collaborative research project for solving global issues”, from the Agriculture, Forestry, and Fisheries Research Council Secretariat, Ministry of Agriculture, Forestry and Fisheries (MAFF), Japan. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Open Access funding provided by Kobe University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikeda, Ki., Uchihashi, K., Okuda, I. et al. Specific detection of Pyricularia oryzae pathotype Triticum using qPCR and LAMP methods. J Gen Plant Pathol 90, 82–94 (2024). https://doi.org/10.1007/s10327-023-01162-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-023-01162-0