Abstract
Phosphorus is an essential element influencing both food security via plant fertilization, and water pollution through excessive phosphorus use, yet the phosphorus cycle in ecosystems is poorly known. In particular, beyond adsorption, the role of iron and manganese oxides in catalyzing the abiotic dephosphorylation of biomolecules is debated. Here, we studied the reactions of ribonucleotides, containing different phosphate bonding, with goethite, hematite, and birnessite. We employed both high-resolution mass spectrometry of solution species and molecular modeling simulations of ribonucleotide-mineral complexes. Results disclose an up to fivefold preferential hydrolytic cleavage of a phosphoanhydride bond over a phosphoester bond, indicating that mineral-catalyzed reactions reflect the hierarchy reported for the activity of phosphatase enzymes. The fourfold higher catalytic reactivity of goethite and birnessite versus hematite is explained by mineral-specific binding rather than surface area differences. Corresponding simulated adsorbate conformations at the water–mineral interfaces are proposed. Overall, our findings provide new insights on the catalytic recycling of organic phosphorus species by mineral oxides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strong binding of phosphorus species to mineral oxides such as iron (hydr)oxides and manganese oxides is important to P sequestration and global P cycling (Sattari et al. 2012; Herndon et al. 2019; Han et al. 2020; Luo et al. 2023). These reactive minerals are proposed to serve as abiotic catalysts in the mineralization of organic phosphorus by facilitating oxidative cleavage of synthetic phosphonates and hydrolytic cleavage of the phosphate moiety in naturally-occurring biomolecules (Nowack and Stone 2003; Huang 2018; Klein et al. 2019; Li et al. 2020; Wan et al. 2022). Understanding adsorption versus catalytic reactivity of minerals towards organic phosphorus is of particular interest, because up to 75% of the phosphorus pool in soils and sediments can be comprised of organic phosphorus compounds (Vincent et al. 2013; McLaren et al. 2015; Ni et al. 2022). There are three common phosphorus bonds in biomolecules that can be involved in mineral-catalyzed hydrolysis: the phosphodiester bond in nucleic acids (Zhang et al. 2023), the phosphoanhydride bond in ribonucleotides (Huang 2018), and the phosphoester bond in sugar phosphates, phytate, and ribonucleotides (Huang 2018; Klein et al. 2019; Wan et al. 2022).
The production of aqueous inorganic phosphate is typically monitored as the only evidence of organic phosphorus dephosphorylation (Wan et al. 2022). Additionally, para-nitrophenyl phosphate, a synthetic phosphomonoester organic phosphorus, has been a convenient assay tool with different colorimetric properties prior to and after dephosphorylation (Baldwin et al. 1995). Mineral-dependent dephosphorylation was observed with para-nitrophenyl phosphate reacted with different iron oxides (Li et al. 2020) and manganese oxides were found to be more reactive than iron oxides towards para-nitrophenyl phosphate (Baldwin et al. 1995). Based on relative production of inorganic phosphate, previous reports have implied different extent of catalytic reactivity of mineral oxides for different types of organic phosphorus (Klein et al. 2019; Wan et al. 2022). Therefore, para-nitrophenyl phosphate is not a suitable analog to evaluate mineral-catalyzed dephosphorylation of naturally-occurring biomolecules.
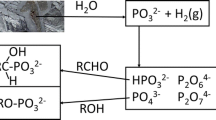
Here we explore the mechanisms of this abiotic dephosphorylation of organic phosphorus by reacting two iron oxides (goethite and hematite) and one manganese oxide (birnessite) with ribonucleotides containing one, two, and three phosphate groups adenosine monophosphate, adenosine diphosphate, and adenosine triphosphate, respectively (Fig. 1a). Ribonucleotides represent important organic phosphorus biomolecules as both breakdown products of nucleic acids, i.e., DNA, RNA, and abundant metabolites in plants and microorganisms (Pietramellara et al. 2009). Importantly, ribonucleotides constitute an important model organic phosphorus to investigate the cleavage of the phosphoanhydride and phosphoester bonds found in the low-molecular-weight organic phosphorus biomolecules considered to be the most susceptible to mineral-catalyzed dephosphorylation.
a Chemical structures of ribonucleotide reactant and dephosphorylation products, and the mass-over-charge ratio (m/z) values used in the liquid chromatography-mass spectrometry analysis. b Calculation of adsorbed inorganic phosphorus (Pi,ads) based on concentration of aqueous species: aqueous inorganic phosphorus (Pi,aq), aqueous adenosine (adenosine,aq), aqueous adenosine monophosphate (AMPaq), and aqueous adenosine diphosphate (ADPaq)
We employed high-resolution liquid chromatography-mass spectrometry to monitor the ribonucleotide reactants and their dephosphorylated organic by-products (Fig. 1a), along with a standard colorimetric technique to quantify aqueous inorganic phosphorus. Previous liquid chromatography-mass spectrometry analysis of the dephosphorylation of adenosine triphosphate by ferrihydrite, another iron oxide, provided evidence of mineral-catalyzed reaction, while inorganic phosphate was absent in solution due to adsorption on the mineral surface (Klein et al. 2019). Here, we leveraged liquid chromatography-mass spectrometry data to obtain a mass balance based on aqueous inorganic phosphate and organic phosphorus species and capture catalytic reactivity that would otherwise be missed (Fig. 1b). We also performed molecular dynamics simulations of ribonucleotide binding to hydrated mineral surfaces to gain insights on the interactions that may serve as precursors to the catalytic process. Our finding shed light on how iron and manganese oxides facilitate abiotic recycling phosphorus from organic phosphorus biomolecules with different phosphorus bonding environments, a process that is not currently considered in the phosphorus cycle.
Experimental
Details on materials, experimental protocols, and computational protocols are reported in the Supplementary Information. Briefly, we performed reactions of each mineral (hematite, goethite, birnessite) with each of the ribonucleotide reactants (adenosine monophosphate, adenosine diphosphate, or adenosine triphosphate) in a carbonate-buffered solution at pH 7.0 with sodium chloride as the background electrolyte. Analysis of the organic species was by high-resolution liquid chromatography-mass spectrometry with orbitrap technology; inorganic phosphate was quantified using the standard molybdate method and spectrophotometry. Molecular modeling of hydrated ribonucleotide-complexes was conducted using established force-fields and molecular dynamics equilibration; parameters for the ribonucleotide structures were obtained by density functional theory calculations. In the Supplementary Information document are the following sections: chemicals and minerals; experiments of ribonucleotide reactions with mineral oxides; analysis of dephosphorylation products; determination of surface area of minerals; determination of inorganic phosphate binding site density on mineral oxides; and, molecular modeling simulations of ribonucleotide adsorption.
Results and discussion
Preferential mineral-catalyzed cleavage of phosphoanhydride bonds over phosphoester bonds in ribonucleotides
For adenosine monophosphate reactions with all the minerals, less than 10% was converted to aqueous inorganic phosphate and lack of aqueous adenosine indicated that the adenosine product remained adsorbed (Fig. 2a). While, no appreciable adenosine monophosphate adsorption was observed with hematite or birnessite, up to 60% of the adenosine monophosphate was adsorbed on goethite (Fig. 2a). Surface-sensitive X-ray absorption spectroscopy has revealed that a fraction (~ 16%) of adenosine monophosphate bound to ferrihydrite was dephosphorylated with the produced inorganic phosphate remaining adsorbed (Klein et al. 2019). Therefore, a small fraction of the goethite-bound adenosine monophosphate may be dephosphorylated.
Evolution of aqueous inorganic phosphorus (Pi, aq), aqueous organic phosphorus, and adsorbed inorganic phosphorus (Pi, ads) species after reactions of 50 µM, a adenosine monophosphate (AMP), b adenosine diphosphate (ADP), and c adenosine triphosphate (ATP) with (from left to right) hematite, goethite, and birnessite for 1 h, 4 h, 1 d, 2 d, and 4 d. In a–c, Pi fractions are shown in the bar graphs (white bars = aqueous Pi; red bars = adsorbed Pi) and aqueous organic phosphorus species are shown in the scatter-and-line plots (gray symbols = aqueous AMP; blue symbols = aqueous ADP; orange symbols = aqueous ATP). In a–c, the connected lines for the aqueous species are to be used as eye guides and are not representative of continuous data as each data point corresponds to a different category specified on the X-axis. The data are provided in Table S1, S2, and S3 in the Supplementary Information files. The data revealed up to fivefold higher fraction of ADP and ATP converted to Pi than AMP, indicating preferential cleavage of phosphoanhydride bonds over phosphoester bonds. More catalytic reactivity with both birnessite and goethite than with hematite implied a role of mineral surface chemistry in dictating reactivity
Compared to adenosine monophosphate, we obtained relatively higher production of inorganic phosphate from adenosine diphosphate and adenosine triphosphate (Fig. 2b, c). While, 12% of adenosine diphosphate-associated phosphorus became inorganic phosphate with hematite, 35–40% was converted to inorganic phosphate with goethite and birnessite; two-thirds of the total inorganic phosphate produced still remained bound on birnessite, whereas two-thirds of the total inorganic phosphate was in solution with goethite, implying a different binding affinity for these two minerals (Fig. 2b). About 50–55% of adenosine triphosphate-associated phosphorus reacted with goethite and birnessite was converted to inorganic phosphate, a near 45% increase compared to adenosine diphosphate reaction (Fig. 2b, c). Interestingly, with adenosine triphosphate, the different fractions of particulate versus aqueous inorganic phosphate were the same as with adenosine diphosphate reacted with goethite and birnessite (Fig. 2c). With these two minerals, aqueous adenosine diphosphate from single dephosphorylation of adenosine triphosphate appeared before adenosine monophosphate; aqueous adenosine monophosphate subsequently accumulated with adenosine diphosphate dephosphorylation (Fig. 2c). This latter trend was not obvious with hematite during the experimental time, but we detected the generation of adenosine diphosphate and minimal adenosine monophosphate in solution (Fig. 2c).
In sum, there was a fivefold preferential cleavage of phosphoanhydride bonds relative to the phosphoester bond in the ribonucleotide reactant, in addition to up to a fourfold higher catalytic reactivity with goethite and birnessite than with hematite (Fig. 2a–c). In agreement with our findings, reactivity based on only aqueous inorganic phosphate production by hematite and birnessite was reported to be up to 30-fold less for phosphomonoesters (glucose-phosphate, glycerophosphate, phytate, and adenosine monophosphate) than for adenosine triphosphate and inorganic polyphosphate, both of which contain phosphoanhydride bonds (Wan et al. 2022). Furthermore, rate constants for adenosine triphosphate and adenosine diphosphate dephosphorylation by a fungal phosphatase were up to three orders of magnitude higher compared to adenosine monophosphate (Solhtalab et al. 2021), indicating that mineral-catalyzed and enzyme-catalyzed dephosphorylation exhibit similar hierarchy in reactivity towards different phosphorus bonding environments. Mineral-dependent dephosphorylation was observed with para-nitrophenyl phosphate, with eightfold greater reactivity with goethite than with hematite, a finding that is consistent with our finding (Li et al. 2020). Moreover, relative to iron oxides, manganese oxides were reported to exhibit about tenfold higher dephosphorylation of para-nitrophenyl phosphate (Baldwin et al. 1995). These previous findings with the synthetic organic phosphorus are consistent with our data, albeit direct comparisons with our reactions with naturally-occurring biomolecules should not be made. For instance, as stated in the aforementioned study (Baldwin et al. 1995), birnessite-mediated dephosphorylation of adenosine triphosphate was tenfold higher than hematite but was comparable to goethite, indicating that birnessite reactivity towards biomolecules may be higher than some but not all iron oxides.
Role of surface area versus binding site density in facilitating catalysis
Due to monitoring both organic and inorganic species on a naturally-occurring organic phosphorus, which is not commonly done in previous studies (Wan et al. 2022), we were able to determine that a significant fraction of the recycled inorganic phosphorus remained bound on the mineral surface. To probe for possible differences in the binding capacity for inorganic phosphate across the different minerals, we determined the surface area of each mineral and performed adsorption experiments to obtain the site density of inorganic phosphate binding for each mineral (Fig. 3a, b). We found that the surface area of birnessite (146.9 m2 g−1) was about ninefold greater than goethite (16 m2 g−1) and nearly 20-fold greater than hematite (7.5 m2 g−1). In regards to the inorganic phosphate binding site density on a per-surface-area basis, birnessite had 3.5-fold and 2.7-fold lower capacity for bound inorganic phosphate than goethite and hematite, respectively (Fig. 3a). This difference implied that, despite their relatively larger surface, birnessite particles exhibited a surface chemistry that facilitated lower density of inorganic phosphate binding sites compared to hematite and goethite. Due to the measurements of catalytic activity in terms of inorganic phosphate produced per mass of mineral, we also determined the inorganic phosphate binding site on a per-mass basis, which revealed that birnessite had 2.8-fold and 7.3-fold greater capacity for bound inorganic phosphate than goethite and hematite, respectively (Fig. 3b). Therefore, the aforementioned threefold higher partitioning of the inorganic phosphate generated from adenosine triphosphate on the birnessite surface than on the goethite surface may be explained by differences in per-mass values of the inorganic phosphate binding site density. After normalization of the final inorganic phosphate production at 4 d by the capacity of each mineral for inorganic phosphate binding on a per-mass basis, there was only a 2.5-fold difference in the dephosphorylation reactivity of adenosine monophosphate or adenosine triphosphate reactivity across the minerals instead of the tenfold difference for the unnormalized values (Fig. 3c). Therefore, the site density for inorganic phosphate binding site could not fully account for the range in mineral reactivity towards the ribonucleotides.
a Surface area-normalized site density for inorganic phosphate binding and b mass-normalized site density for inorganic phosphate binding on hematite (white bars), goethite (gray bars), and birnessite (black bars). c Total produced inorganic phosphate normalized by inorganic phosphate binding site density after 4-d reaction of adenosine monophosphate (AMP, left) and adenosine triphosphate (ATP, right) with hematite (white bars), goethite (gray bars), and birnessite (black bars). Statistical analysis of differences was performed using data from three independent replicated at each condition: ns (not significant), *(p < 0.05), **(p < 0.01), ***(p < 0.001). The data are provided in Table S4 and S5 in the Supplementary Information files. The inorganic phosphate binding density accounted, to a large extent, for the mineral-dependent differences in reactivity. We proposed that other surface chemistry characteristics such as availability of Lewis acid sites may also play a role in facilitating the extent of reactivity of each mineral
Molecular modeling simulation of binding conformations at the water–mineral interface
The hierarchy in enzymatic dephosphorylation activity towards different organic phosphorus biomolecules has been attributed to more favorable binding of the terminal phosphate in the active site (Solhtalab et al. 2021), which can involve manganese and iron centers in plant phosphatases (Schenk et al. 2005). Exposed facets in hematite and goethite with nearly twofold higher Lewis acid sites had up to twofold more para-nitrophenyl phosphate dephosphorylation (Li et al. 2020). Binding coordination at Lewis acid sites was proposed to catalyze hydrolysis, through either weakening of the phosphoester bond due to electron transfer from a bound phosphate to an iron center or the nucleophilic attack of the phosphoester bond by an activated bound water (Fang et al. 2018; Li et al. 2020). From molecular simulations of a dry system, it was proposed that binding conformations of the adsorbed ribonucleotides may dictate the extent of dephosphorylation by ferrihydrite (Klein et al. 2019). Taken collectively, these previous studies highlight the importance of binding of the phosphate moiety as well as the conformation of the bound organic phosphorus to generate a favorable pre-catalytic complex of organic phosphorus with the mineral to facilitate the dephosphorylation reaction.
Here, we performed molecular dynamics simulations of the interactions of adenosine monophosphate and adenosine triphosphate with goethite in a hydrated system with electrolytes to gain preliminary insights on this pre-catalytic complex (Fig. 4a). Following thermodynamic and geometry optimizations, the optimized structures of the adsorbed ribonucleotides revealed water-bridged interactions with only the phosphate moiety in the complex of adenosine triphosphate with goethite, whereas water-bridged interactions involved both phosphate and ribose moieties in the complex of adenosine monophosphate with goethite (Fig. 4a, b). Additionally, the sodium ions complexed by phosphate oxygens in the ribonucleotide were coordinated to the mineral surface both directly to mineral oxygens and indirectly via water bridges (Fig. 4a, b). Therefore, our molecular modeling implied that bridging interactions between the mineral surface with both phosphate and sugar moieties of adenosine monophosphate would impede favorable orientation for catalysis and that water-bridged interactions would be important in mediating the hydrolytic cleavage for both adenosine monophosphate and adenosine triphosphate.
a Configurations of (left) adenosine monophosphate (AMP) and (right) adenosine triphosphate (ATP) in the bulk solution prior to binding at the water-goethite interface. Optimized adsorbate conformations of (b) adenosine monophosphate and (c) adenosine triphosphate at the water-goethite interface. In b and c, only waters involved in bridging are shown for clarity. Color code in A and B: water (light blue), oxygen (red), hydrogen (white), nitrogen (dark blue), sodium (green), phosphorus (pink), iron (purple polyhedron); water bridging (blue dotted line) and metal coordination (red dotted line) interactions. The starting model configurations are provided in the Supplementary Information files. The optimized configurations from the molecules simulations revealed that water-bridged interactions only involved the phosphate moiety in the complex of adenosine triphosphate with goethite but water-bridged interactions involved both phosphate and ribose moieties in the complex of adenosine monophosphate with goethite, implying different binding conformations dictated different extent of reactivity
Our theoretical configurations of hydrated ribonucleotide–mineral complexes thus highlighted both specific adsorbate conformation and involvement of bound water, presenting for the first time that aspects of both previously proposed mechanisms could be simultaneously important in mediating the abiotic catalysis of organic phosphorus recycling by the mineral oxides (López et al. 2021). In light of our findings, we propose that future mechanistic investigations consider the number and strength of Lewis acid sites in the mineral structure (Fang et al. 2020; Li et al. 2020), hydroxyls and oxygens on the mineral surfaces (Barrón and Torrent 1996), and conformations of organic phosphorus binding at the water–mineral interfaces of iron oxides and manganese oxides (Han et al. 2020).
Conclusion
This research advances structural considerations in the evaluation of phosphorus recycling from environmentally relevant organic phosphorus catalyzed by different iron oxide and manganese oxide surfaces. We reveal that organic phosphorus biomolecules with phosphoanhydride bonds are fivefold more susceptible to mineral-catalyzed dephosphorylation than phosphoester bonds. A fourfold difference in the reactivity across the different mineral oxides seemed to be dependent on both the chemistry and structure of the mineral surface. Importantly, the capacity of the mineral surface to trap the resulting inorganic phosphate must be accounted for in predicting the fate of mineral-catalyzed recycling inorganic phosphate from organic phosphorus biomolecules. Finally, quantitative accounting of this abiotic catalysis in environmental matrices is required to inform its contribution to the global phosphorus cycling, an important biogeochemical cycle intimately linked to carbon cycling.
Availability of data and material
The data are made available in Supplementary files.
References
Baldwin DS, Beattie JK, Coleman LM, Jones DR (1995) Phosphate ester hydrolysis facilitated by mineral phases. Environ Sci Technol 29:1706–1709. https://doi.org/10.1021/es00006a040
Barrón V, Torrent J (1996) Surface hydroxyl configuration of various crystal faces of hematite and goethite. J Colloid Interface Sci 177:407–410. https://doi.org/10.1006/jcis.1996.0051
Fang Y, Kim E, Strathmann TJ (2018) Mineral- and base-catalyzed hydrolysis of organophosphate flame retardants: potential major fate-controlling sink in soil and aquatic environments. Environ Sci Technol 52:1997–2006. https://doi.org/10.1021/acs.est.7b05911
Fang Y, Cao X, Feng W et al (2020) High catalytic hydrolysis of microcystins on pyrite surface. Environ Chem Lett 18:483–487. https://doi.org/10.1007/s10311-019-00948-z
Han J, Kim M, Ro H-M (2020) Factors modifying the structural configuration of oxyanions and organic acids adsorbed on iron (hydr)oxides in soils. A review. Environ Chem Lett 18:631–662. https://doi.org/10.1007/s10311-020-00964-4
Herndon EM, Kinsman-Costello L, Duroe KA et al (2019) Iron (Oxyhydr)Oxides serve as phosphate traps in tundra and boreal peat soils. J Geophys Res Biogeosci 124:227–246. https://doi.org/10.1029/2018JG004776
Huang X-L (2018) Hydrolysis of phosphate esters catalyzed by inorganic iron oxide nanoparticles acting as biocatalysts. Astrobiology. https://doi.org/10.1089/ast.2016.1628
Klein AR, Bone SE, Bakker E et al (2019) Abiotic phosphorus recycling from adsorbed ribonucleotides on a ferrihydrite-type mineral: Probing solution and surface species. J Colloid Interface Sci 547:171–182. https://doi.org/10.1016/j.jcis.2019.03.086
Li T, Zhong W, Jing C et al (2020) Enhanced hydrolysis of p-nitrophenyl phosphate by iron (Hydr)oxide nanoparticles: roles of exposed facets. Environ Sci Technol 54:8658–8667. https://doi.org/10.1021/acs.est.9b07473
López YC, Viltres H, Gupta NK et al (2021) Transition metal-based metal–organic frameworks for environmental applications: a review. Environ Chem Lett 19:1295–1334. https://doi.org/10.1007/s10311-020-01119-1
Luo D, Wang L, Nan H et al (2023) Phosphorus adsorption by functionalized biochar: a review. Environ Chem Lett 21:497–524. https://doi.org/10.1007/s10311-022-01519-5
McLaren TI, Smernik RJ, McLaughlin MJ et al (2015) Complex forms of soil organic phosphorus—a major component of soil phosphorus. Environ Sci Technol 49:13238–13245. https://doi.org/10.1021/acs.est.5b02948
Ni Z, Li Y, Wang S (2022) Cognizing and characterizing the organic phosphorus in lake sediments: advances and challenges. Water Res 220:118663. https://doi.org/10.1016/j.watres.2022.118663
Nowack B, Stone AT (2003) Manganese-catalyzed degradation of phosphonic acids. Environ Chem Lett 1:24–31. https://doi.org/10.1007/s10311-002-0014-3
Pietramellara G, Ascher J, Borgogni F et al (2009) Extracellular DNA in soil and sediment: fate and ecological relevance. Biol Fertil Soils 45:219–235. https://doi.org/10.1007/s00374-008-0345-8
Sattari SZ, Bouwman AF, Giller KE, van Ittersum MK (2012) Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc Natl Acad Sci 109:6348–6353. https://doi.org/10.1073/pnas.1113675109
Schenk G, Gahan LR, Carrington LE et al (2005) Phosphate forms an unusual tripodal complex with the Fe–Mn center of sweet potato purple acid phosphatase. Proc Natl Acad Sci U S A 102:273–278. https://doi.org/10.1073/pnas.0407239102
Solhtalab M, Klein AR, Aristilde L (2021) Hierarchical reactivity of enzyme-mediated phosphorus recycling from organic mixtures by Aspergillus niger phytase. J Agric Food Chem 69:2295–2305. https://doi.org/10.1021/acs.jafc.0c05924
Vincent AG, Vestergren J, Gröbner G et al (2013) Soil organic phosphorus transformations in a boreal forest chronosequence. Plant Soil 367:149–162. https://doi.org/10.1007/s11104-013-1731-z
Wan B, Huang R, Diaz JM, Tang Y (2022) Rethinking the biotic and abiotic remineralization of complex phosphate molecules in soils and sediments. Sci Total Environ 833:155187. https://doi.org/10.1016/j.scitotenv.2022.155187
Zhang K, Ho K-P, Chatterjee A et al (2023) RNA hydrolysis at mineral-water interfaces. Environ Sci Technol 57:8280–8288. https://doi.org/10.1021/acs.est.3c01407
Acknowledgements
We are grateful to Dr Geunho Han from the Lab of Dr Justin Notestein at Northwestern University for performing the measurements to obtain the surface area of the minerals.
Funding
This research was funded by the U.S. Department of Energy (Geosciences Program, DE-SC0021172).
Author information
Authors and Affiliations
Contributions
L.A. supervised research. A.R.K., J.J.B., and L.A. designed research. A.R.K., J.J.B., A.N.M, C.X.C., M.S., and W.T. performed research and analyzed data. J.J.B., A.N.M., and L.A. wrote manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare they have no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klein, A.R., Basinski, J.J., Niyitanga Manzi, A. et al. Phosphorus recycling by mineral-catalyzed ribonucleotide cleavage on iron and manganese oxides. Environ Chem Lett (2024). https://doi.org/10.1007/s10311-024-01754-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10311-024-01754-y