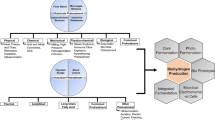
Abstract
The negative effects of the accelerating climate change due partly to fossil fuel consumption is calling for the rapid development of sustainable energies such as biohydrogen, which is produced using microorganisms. Here we review biohydrogen production from biomass, with focus on biomass pretreatment, fermentative production, factors affecting production, bioreactors, kinetics and modeling, and improved production with nanoparticles. Pretreatments include chemical, physical and biological methods. Hydrogen production is done by photo-fermentation or dark fermentation. Influencing factors comprise pH, temperature, hydraulic retention time, and the presence of fermentation inhibitors. Continuous stirred tank-, anaerobic fluidized bed-, anaerobic sequencing batch-, up-flow anaerobic sludge blanket- and dynamic membrane reactors are used. Additives include cobalt, nickel and iron nanoparticles. Compared to thermochemical, photochemical and electrochemical processes, biohydrogen production needs more time but is easy to operate, cost-effective and environmentally friendly.












Similar content being viewed by others
Abbreviations
- %:
-
Percentage
- °C/min:
-
Degree Celsius per minute
- °C:
-
Degree Celsius
- atm:
-
Atmosphere (unit of pressure)
- ADP:
-
Adenosine diphosphate
- ATP:
-
Adenosine triphosphate
- BGU/mL:
-
β-Glucanase units per milliliter
- CH4 :
-
Methane
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- COD:
-
Chemical oxygen demand
- d:
-
Day
- e– :
-
Electron
- Fe:
-
Iron
- FPU/mL:
-
Filter paper units per milliliter
- G(t):
-
Cumulative CH4 or H2 production
- g:
-
Grams
- Gt CO2-eq/year:
-
Gigaton carbon dioxide equivalent per year
- H+ :
-
Proton
- h:
-
Hours
- H2 :
-
Hydrogen
- H2O:
-
Water
- H2O2 :
-
Hydrogen peroxide
- H2SO4 :
-
Sulfuric acid
- H3PO4 :
-
Phosphoric acid
- HCl:
-
Hydrochloric acid
- IU/mL:
-
International units per milliliter
- J:
-
Instantaneous flux
- kg:
-
Kilogram
- kHz:
-
Kilohertz
- kJ/mol:
-
Kilojoule per mole
- KOH:
-
Potassium hydroxide
- kPa:
-
Kilopascal
- kW:
-
Kilowatt
- L:
-
Liter
- M:
-
Molar
- m2 :
-
Meter Square
- m3 :
-
Cubic meter
- mg:
-
Milligram
- min:
-
Minute
- MJ/kg:
-
Megajoule per kilogram
- mL:
-
Microliter
- mM:
-
Millimole
- mol/mol:
-
Mole per mole
- N:
-
Normal
- N2 :
-
Nitrogen
- NAD:
-
Nicotinamide adenine dinucleotide
- NADH:
-
Nicotinamide adenine dinucleotide + hydrogen
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate hydrogen
- NaOH:
-
Sodium hydroxide
- Ni:
-
Nickel
- O2 :
-
Oxygen
- P:
-
Maximum CH4 or H2 production potential
- Pa:
-
Pascal
- Pa·s:
-
Pascal-second
- psi:
-
Pound per square inch
- Rc :
-
Cake layer resistance
- Rm :
-
Intrinsic resistance of membrane mesh
- Rmax :
-
Maximum CH4 or H2 production rate
- Rp :
-
Pore-clogging resistance
- rpm:
-
Revolutions per minute
- Rt :
-
Total membrane resistance
- s:
-
Seconds
- S:
-
Sulfur
- t:
-
Time
- TMP:
-
Trans-membrane pressure
- U/L:
-
Units per liter
- V:
-
Volt
- vol.%:
-
Volume percent
- w:
-
Watt
- wt.%:
-
Weight percentage
- λ:
-
Lag phase
- μ:
-
Dynamic viscosity of permeate water
References
Abdolmaleki A, Nabavizadeh SS, Badbedast M (2021) 1-(Carboxymethyl)pyridinium chloride as an acidic ionic liquid for rice straw effective pretreatment. Renew Energy 177:544–553. https://doi.org/10.1016/j.renene.2021.05.158
Abud AKS, Amorim ELC (2021) Evaluation of biohydrogen production from sugarcane vinasse in an anaerobic fluidized bed reactor without pH control. Latin Am Appl Res 51:63–69
Adessi A, Venturi M, Candeliere F, Galli V, Granchi L, De Philippis R (2018) Bread wastes to energy: sequential lactic and photo-fermentation for hydrogen production. Int J Hydrogen Energy 43:9569–9576. https://doi.org/10.1016/j.ijhydene.2018.04.053
Ahmar M, Parnthong J, Kungsanant S, Chavadej S, Chaiprapat S (2022) Influences of specific surfactant structures on biohydrogen production from oily wastewater in batch and continuous anaerobic dark fermentation. Bioresour Technol 360:127617. https://doi.org/10.1016/j.biortech.2022.127617
Alejandro P, Orfila M, Linares M, Raúl S, Javier M, Raúl M, Juan A (2022) Hydrogen production by thermochemical water splitting with La0.8Al0.2MeO3-δ (Me = Fe Co, Ni and Cu) perovskites prepared under controlled pH. Catal Today 391:22–33. https://doi.org/10.1016/j.cattod.2021.12.014
Al-Mohammedawi HH, Znad H, Eroglu E (2019) Improvement of photofermentative biohydrogen production using pre-treated brewery wastewater with banana peels waste. Int J Hydrogen Energy 44:2560–2568. https://doi.org/10.1016/j.ijhydene.2018.11.223
Balakrishnan D, Manmai N, Ponnambalam S, Yuwalee U, Chudapak C, Rameshprabu R (2023) Optimized model of fermentable sugar production from Napier grass for biohydrogen generation via dark fermentation. Int J Hydrogen Energy 48:21152–21160. https://doi.org/10.1016/j.ijhydene.2022.12.011
Banoth C, Sunkar B, Raj P, Bhukya B (2017) Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 7:334. https://doi.org/10.1007/s13205-017-0980-6
Banu JR, Kavitha S, Kannah RY, Usman TMM (2020) Application of chemo thermal coupled sonic homogenization of marine macroalgal biomass for energy efficient volatile fatty acid recovery. Bioresour Technol 303:122951. https://doi.org/10.1016/j.biortech.2020.122951
Banu JR, Kavitha S, Kannah Y, Tyagi V, Kumar G (2023) Combined sodium citrate and ultrasonic pretreatment of waste activated sludge for cost effective production of biogas. Bioresour Technol 376:128857. https://doi.org/10.1016/j.biortech.2023.128857
Barros AR, Adorno MAT, Sakamoto IK, Maintinguer SI, Varesche MBA, Silv EL (2011) Performance evaluation and phylogenetic characterization of anaerobic fluidized bed reactors using ground tire and pet as support materials for biohydrogen production. Bioresour Technol 102:3840–3847. https://doi.org/10.1016/j.biortech.2010.12.014
Baykara SZ (2018) Hydrogen: a brief overview on its sources, production and environmental impact. Int J Hydrogen Energy 43:10605–10614. https://doi.org/10.1016/j.ijhydene.2018.02.022
Borges T, Catucci G, Rodrigues L, Camila A, Soares A, Sakamoto I, Bernadete I, Silva EL (2019) HRT control as a strategy to enhance continuous hydrogen production from sugarcane juice under mesophilic and thermophilic conditions in AFBRs. Int J Hydrogen Energy 44:19719–19729. https://doi.org/10.1016/j.ijhydene.2019.06.050
Braga AFM, Lens PNL (2023) Natural fermentation as an inoculation strategy for dark fermentation of Ulva spp. hydrolysate. Biomass Bioenergy 176:106902. https://doi.org/10.1016/j.biombioe.2023.106902
Brindha K, Mohanraj S, Rajaguru P, Pugalenthi V (2023) Simultaneous production of renewable biohydrogen, biobutanol and biopolymer from phytogenic CoNPs-assisted Clostridial fermentation for sustainable energy and environment. Sci Total Environ 859:160002. https://doi.org/10.1016/j.scitotenv.2022.160002
Bu J, Wei H, Wang Y, Cheng J, Zhu M (2021) Biochar boosts dark fermentative H2 production from sugarcane bagasse by selective enrichment/colonization of functional bacteria and enhancing extracellular electron transfer. Water Res 202:117440. https://doi.org/10.1016/j.watres.2021.117440
Bui VD, Vu HP, Nguyen HP, Duong XQ, Nguyen DT, Pham MT, Nguyen PQP (2023) Techno-economic assessment and logistics management of biomass in the conversion progress to bioenergy. Sustain Energy Technol Assess 55:102991. https://doi.org/10.1016/j.seta.2022.102991
Camus P, Mehanovic D, Castellanos-beltran IJ, Dufault J, Camus P, Castellanos-Beltran IJ, Braidy N, Frechette LG, Picard M (2023) Electrified steam methane reforming microreactor. Int J Hydrogen Energy 49:907–915. https://doi.org/10.1016/j.ijhydene.2023.07.343
Cesaro A, Belgiorno V (2014) Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem Eng J 240:24–37. https://doi.org/10.1016/j.cej.2013.11.055
Cesaro A, Conte A, Trably E, Carrere H, Trably E, Paillet F, Belgiorno V (2020) Formic acid pretreatment for enhanced production of bioenergy and biochemicals from organic solid waste. Biomass Bioenerg 133:105455. https://doi.org/10.1016/j.biombioe.2019.105455
Chandrasekhar K, Mehrez I, Kumar G, Kim SH (2021) Relative evaluation of acid, alkali, and hydrothermal pretreatment influence on biochemical methane potential of date biomass. J Environ Chem Eng 9:106031. https://doi.org/10.1016/j.jece.2021.106031
Chavan S, Gaikwad A (2023) Hydrogen generation from bamboo biomass using enzymatic hydrolysis and subsequent microbial electrolysis in a single chamber microbial electrolysis cell. Biochem Eng J 193:108853. https://doi.org/10.1016/j.bej.2023.108853
Chen CY, Yang MH, Yeha KL, Liua CH, Chang JS (2008) Biohydrogen production using sequential two-stage dark and photo fermentation processes. Int J Hydrogen Energy 33:4755–4762. https://doi.org/10.1016/j.ijhydene.2008.06.055
Chen D, Kuang Y, Wang H, Liang J, Zhao J (2022) Insights into the mechanism of naproxen inhibiting biohydrogen production from sludge dark fermentation. Process Saf Environ Prot 167:390–397. https://doi.org/10.1016/j.psep.2022.09.015
Cheng J, Xia A, Su H, Song W, Zhou J, Cen K (2013) Promotion of H2 production by microwave-assisted treatment of water hyacinth with dilute H2SO4 through combined dark fermentation and photofermentation. Energy Convers Manag 73:329–334. https://doi.org/10.1016/j.enconman.2013.05.018
Cheng J, Li H, Ding L, Zhoua J, Songb W, Lic YY, Lind R (2020) Improving hydrogen and methane co-generation in cascading dark fermentation and anaerobic digestion: the effect of magnetite nanoparticles on microbial electron transfer and syntrophism. Chem Eng J 397:125394. https://doi.org/10.1016/j.cej.2020.125394
Cieciura-Włoch W, Borowski S, Otlewska A (2020) Biohydrogen production from fruit and vegetable waste, sugar beet pulp and corn silage via dark fermentation. Renew Energy 153:1226–1237. https://doi.org/10.1016/j.renene.2020.02.085
Corneli E, Adessi A, Dragoni F et al (2016) Agroindustrial residues and energy crops for the production of hydrogen and poly-β-hydroxybutyrate via photofermentation. Bioresour Technol 216:941–947. https://doi.org/10.1016/j.biortech.2016.06.046
D’ Silva TC, Isha A, Chandra R, Vijay VK, Subbarao PMV, Kumar R, Chaudhary VP, Singh H, Khan AA, Tyagi VK, Kovács KL (2021) Enhancing methane production in anaerobic digestion through hydrogen assisted pathways – a state-of-the-art review. Renew Sustain Energy Rev 151:111536. https://doi.org/10.1016/j.rser.2021.111536
da Silva AN, Macêdo WV, Sakamoto IK, Pereyraa DAD, Mendesa CO, Maintinguerb SI, Filhoa RAC, Damianovicc MHZ, Varescheb MBA, Amorima ELC (2019) Biohydrogen production from dairy industry wastewater in an anaerobic fluidized-bed reactor. Biomass Bioenergy 120:257–264. https://doi.org/10.1016/j.biombioe.2018.11.025
Devriendt MFSN, Guisson BWR, Erik JKB (2022) Techno-economic feasibility of extrusion as a pretreatment step for biogas production from grass. BioEnergy Res 15:1232–1239. https://doi.org/10.1007/s12155-021-10287-z
Duque A, Manzanares P, Ballesteros M (2017) Extrusion as a pretreatment for lignocellulosic biomass: fundamentals and applications. Renew Energy 114:1427–1441. https://doi.org/10.1016/j.renene.2017.06.050
Dutta N, Usman M, Ashraf MA, Luo G, El-Din MG, Zhang S (2023) Methods to convert lignocellulosic waste into biohydrogen, biogas, bioethanol, biodiesel and value-added chemicals: a review. Environ Chem Lett 21:803–820. https://doi.org/10.1007/s10311-022-01511-z
Ebrahimian F, Karimi K (2020) Efficient biohydrogen and advanced biofuel coproduction from municipal solid waste through a clean process. Bioresour Technol 300:122656. https://doi.org/10.1016/j.biortech.2019.122656
Elgarahy AM, Eloffy MG, Hammad A, Saber AM, El-Sheri DM, Mohsen A, Abouzid M, Elwakeel MZ (2022) Hydrogen production from wastewater, storage, economy, governance and applications: a review. Environ Chem Lett 20:3453–3504. https://doi.org/10.1007/s10311-022-01480-3
Elsamadony M, Tawfik A, William AD, Suzuki M (2015) Optimization of hydrogen production from organic fraction of municipal solid waste (OFMSW) dry anaerobic digestion with analysis of microbial community. Int J Energy Res 39:929–940. https://doi.org/10.1002/er.3297
Estévez S, Rebolledo-Leiva R, Hernández D, González-García S, Feijoo G, Moreira MT (2023) Benchmarking composting, anaerobic digestion and dark fermentation for apple vinasse management as a strategy for sustainable energy production. Energy 274:127319. https://doi.org/10.1016/j.energy.2023.127319
Fasheun DO, da Silva AS, Teixeira RSS, Ferreira-Leitão VS (2024) Dark fermentative hydrogen production from cassava starch: a comprehensive evaluation of the effects of starch extrusion and enzymatic hydrolysis. Int J Hydrogen Energy 52:322–334. https://doi.org/10.1016/j.ijhydene.2023.05.312
Fdez-Güelfo LA, Álvarez-Gallego C, Márquez DS, García LIR (2011) Biological pretreatment applied to industrial organic fraction of municipal solid wastes (OFMSW): effect on anaerobic digestion. Chem Eng J 172:321–325. https://doi.org/10.1016/j.cej.2011.06.010
Franco-León J de J, Arriola-Guevara E, Suárez-Hernández LA, Toriz G, Guatemala-Morales G, Corona- González RI (2021) Influence of supplemented nutrients in tequila vinasses for hydrogen and polyhydroxybutyrate production by photofermentation with Rhodopseudomonas pseudopalustris. Bioresour Technol 329:124865. https://doi.org/10.1016/j.biortech.2021.124865
Gadhe A, Sonawane SS, Varma MN (2015) Enhancement effect of hematite and nickel nanoparticles on biohydrogen production from dairy wastewater. Int J Hydrogen Energy 40:4502–4511. https://doi.org/10.1016/j.ijhydene.2015.02.046
Ganeshan P, Vigneswaran VS, Gowd SC, Kondusamy D, Kumar CS, Krishnamoorthy N, Kumar D, Juneja A, Paramasivan B, Raju NN, Rajendran K, Pugazhendhi A (2023) How does techno-economic analysis and lifecycle assessment help in commercializing the biohydrogen supply chain? Fuel 341:127601. https://doi.org/10.1016/j.fuel.2023.127601
Gao R, Guo X, Liu S, Liu S, Zhang X, Liu X, Su Y, Wang S (2022) Ultrastable and high-performance seawater-based photoelectrolysis system for solar hydrogen generation. Appl Catal B Environ 304:120883. https://doi.org/10.1016/j.apcatb.2021.120883
García-Depraect O, Rene ER, Diaz-Cruces VF, León-Becerril E (2019) Effect of process parameters on enhanced biohydrogen production from tequila vinasse via the lactate-acetate pathway. Bioresour Technol 273:618–626. https://doi.org/10.1016/j.biortech.2018.11.056
Ghavam S, Vahdati M, Wilson IAG, Styring P (2021) Sustainable ammonia production processes. Front Energy Res 9:1–19. https://doi.org/10.3389/fenrg.2021.580808
Gielen D, Boshell F, Saygin D, Bazilianc MD, Wagnera N, Gorinia R (2019) The role of renewable energy in the global energy transformation. Energy Strat Rev 24:38–50. https://doi.org/10.1016/j.esr.2019.01.006
Gomes SD, Fuess LT, Penteado ED, Lucas SHM, Gotardo JT, Zaiat M (2015) The application of an innovative continuous multiple tube reactor as a strategy to control the specific organic loading rate for biohydrogen production by dark fermentation. Bioresour Technol 197:201–207. https://doi.org/10.1016/j.biortech.2015.08.077
González-Arias J, Zhang Z, Reina TR, Odriozola JA (2023) Hydrogen production by catalytic aqueous-phase reforming of waste biomass: a review. Environ Chem Lett 21:3089–3104. https://doi.org/10.1007/s10311-023-01643-w
Gorgec FK, Karapinar I (2019) Biohydrogen production from hydrolyzed waste wheat by dark fermentation in a continuously operated packed bed reactor: the effect of hydraulic retention time. Int J Hydrogen Energy 44:136–143. https://doi.org/10.1016/j.ijhydene.2018.08.155
Gray V (2007) Climate change 2007: the physical science basis summary for policymakers. Energy Environ 18:433–440. https://doi.org/10.1260/095830507781076194
Greses S, Tomás-Pejó E, González-Fernández C (2021) Short-chain fatty acids and hydrogen production in one single anaerobic fermentation stage using carbohydrate-rich food waste. J Clean Prod 284:124727. https://doi.org/10.1016/j.jclepro.2020.124727
Guan X, Sun Y, Qin H, Li J, Lo IMC, He D, Dong H (2015) The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: the development in zero-valent iron technology in the last two decades (1994–2014). Water Res 75:224–248. https://doi.org/10.1016/j.watres.2015.02.034
Guiao KS, Gupta A, Tzoganakis C, Mekonnen TH (2022) Reactive extrusion as a sustainable alternative for the processing and valorization of biomass components. J Clean Prod 355:131840. https://doi.org/10.1016/j.jclepro.2022.131840
Han W, Huang J, Zhao H, Li Y (2016) Continuous biohydrogen production from waste bread by anaerobic sludge. Bioresour Technol 212:1–5. https://doi.org/10.1016/j.biortech.2016.04.007
Hay JXW, Wu TY, Juan JC, Md. Jahim J (2017) Effect of adding brewery wastewater to pulp and paper mill effluent to enhance the photofermentation process: wastewater characteristics, biohydrogen production, overall performance, and kinetic modeling. Environ Sci Pollut Res 24:10354–10363. https://doi.org/10.1007/s11356-017-8557-9
Hellal MS, Gamon F, Cema G, Hassan GK, Mohamed GG, Ziembińska-Buczyńska A (2024) Nanoparticle-assisted biohydrogen production from pretreated food industry wastewater sludge: microbial community shifts in batch and continuous processes. Energy Convers Manag 299:117824. https://doi.org/10.1016/j.enconman.2023.117824
Hitit ZY, Zampol Lazaro C, Hallenbeck PC (2017) Increased hydrogen yield and COD removal from starch/glucose based medium by sequential dark and photo-fermentation using Clostridium butyricum and Rhodopseudomonas palustris. Int J Hydrogen Energy 42:18832–18843. https://doi.org/10.1016/j.ijhydene.2017.05.161
Hu B, Li Y, Zhu S, Zhang H, Jing Y, Jiang D, He C, Zhang Z (2020) Evaluation of biohydrogen yield potential and electron balance in the photo-fermentation process with different initial pH from starch agricultural leftover. Bioresour Technol 305:122900. https://doi.org/10.1016/j.biortech.2020.122900
Husna A, Aziz A, Sakinah N, Mansor MF, Abdul PM, Arisht SN, Jamali NS, Tiang MF (2022) Synergistic enhancement of biohydrogen production by supplementing with green synthesized magnetic iron nanoparticles using thermophilic mixed bacteria culture. Int J Hydrogen Energy 47:40683–40695. https://doi.org/10.1016/j.ijhydene.2022.09.105
Isiaka A, Johan P, Erasmus M (2023) Reports from garbage to treasure: a review on biorefinery of organic solid wastes into valuable biobased products. Bioresour Technol Rep 24:101610. https://doi.org/10.1016/j.biteb.2023.101610
Izzi AZ, Wan Ishak WMF, Yusuf NNAN, Alenezib RA, Alias NA (2023) Thermophilic biohydrogen production from optimized enzymatic pretreatment of palm oil mill effluent via Box-Behnken design. J Eng Res 11:100054. https://doi.org/10.1016/j.jer.2023.100054
Jamali NS, Md Jahim J, Wan Isahak WNR (2016) Biofilm formation on granular activated carbon in xylose and glucose mixture for thermophilic biohydrogen production. Int J Hydrogen Energy 41:21617–21627. https://doi.org/10.1016/j.ijhydene.2016.05.092
Jha S, Nanda S, Acharya B, Dalai AK (2022a) A review of thermochemical conversion of waste biomass to biofuels. Energies 15:6352. https://doi.org/10.3390/en15176352
Jha S, Okolie JA, Nanda S, Dalai AK (2022b) A review of biomass resources and thermochemical conversion technologies. Chem Eng Technol 45:791–799. https://doi.org/10.1002/ceat.202100503
Ji J, Cai Z, Shen L (2024) Comprehensive insight into synergistic enhancement of nickel and iron doped lanthanum manganese oxide for biohydrogen production via anaerobic fermentation. Fuel 357:129974. https://doi.org/10.1016/j.fuel.2023.129974
Jiang C, Geng J, Hu H, Ma H, Gao X, HeT R (2017) Impact of selected non-steroidal anti-inflammatory pharmaceuticals on microbial community assembly and activity in sequencing batch reactors. PLoS ONE 12:1–16. https://doi.org/10.1371/journal.pone.0179236
Jihen T, Baligh M, Amel F, Said N, Moktar H, Maher G, Hassib B (2015) Microbial ecology overview during anaerobic codigestion of dairy wastewater and cattle manure and use in agriculture of obtained bio-fertilisers. Bioresour Technol 198:141–149. https://doi.org/10.1016/j.biortech.2015.09.004
Jin X, Wei S (2023) Heliyon efficient short time pretreatment on lignocellulosic waste using an isolated fungus Trametes sp. W-4 for the enhancement of biogas production. Heliyon 9:e14573. https://doi.org/10.1016/j.heliyon.2023.e14573
Jung KW, Cho SK, Yun YM, Shin HS, Kim DH (2013) Rapid formation of hydrogen-producing granules in an up-flow anaerobic sludge blanket reactor coupled with high-rate recirculation. Int J Hydrogen Energy 38:9097–9103. https://doi.org/10.1016/j.ijhydene.2013.05.059
Kaloudas D, Pavlova N, Penchovsky R (2021) Lignocellulose, algal biomass, biofuels and biohydrogen: a review. Environ Chem Lett 19:2809–2824. https://doi.org/10.1007/s10311-021-01213-y
Karthikeyan B, Gokuladoss V (2022) Fusion of vermicompost and sewage sludge as dark fermentative biocatalyst for biohydrogen production: a kinetic study. Energies 15:6917. https://doi.org/10.3390/en15196917
Kavitha S, Banu JR, Kumar G, Kaliappanc S, Yeom IT (2018) Profitable ultrasonic assisted microwave disintegration of sludge biomass: modelling of biomethanation and energy parameter analysis. Bioresour Technol 254:203–213. https://doi.org/10.1016/j.biortech.2018.01.072
Kavitha S, Gondi R, Kannah RY, Kumar G, Banu JR (2023) A review on current advances in the energy and cost effective pretreatments of algal biomass: enhancement in liquefaction and biofuel recovery. Bioresour Technol 369:128383. https://doi.org/10.1016/j.biortech.2022.128383
Keskin T, Hallenbeck PC (2012) Hydrogen production from sugar industry wastes using single-stage photofermentation. Bioresour Technol 112:131–136. https://doi.org/10.1016/j.biortech.2012.02.077
Khan ST (2022) Consortia-based microbial inoculants for sustaining agricultural activities. Appl Soil Ecol 176:104503. https://doi.org/10.1016/j.apsoil.2022.104503
Khan S, Nisar A, Wu B, Zhu QL, Wang YW, Hu GQ, He M (2022) Bioenergy production in Pakistan: potential, progress, and prospect. Sci Total Environ 814:152872. https://doi.org/10.1016/j.scitotenv.2021.152872
Khandelwal K, Nanda S, Boahene P, Dalai AK (2023) Conversion of biomass into hydrogen by supercritical water gasification: a review. Environ Chem Lett 21:1–20. https://doi.org/10.1007/s10311-023-01624-z
Khor WC, Vervaeren H, Rabaey K (2018) Combined extrusion and alkali pretreatment improves grass storage towards fermentation and anaerobic digestion. Biomass Bioenergy 119:121–127. https://doi.org/10.1016/j.biombioe.2018.09.003
Kucharska K, Cieśliński H, Rybarczyk P, Słupek E, Łukajtis R, Wychodnik K, Kaminski M (2019) Fermentative conversion of two-step pre-treated lignocellulosic biomass to hydrogen. Catalysts 9:858. https://doi.org/10.3390/catal9100858
Kumar G, Bakonyi P, Kobayashi T, Xu KQ, Sivagurunathan P, Kim SH, Buitrón G, Nemestóthy N, Bakó KB (2016) Enhancement of biofuel production via microbial augmentation: the case of dark fermentative hydrogen. Renew Sustain Energy Rev 57:879–891. https://doi.org/10.1016/j.rser.2015.12.107
Kumar D, Eswari AP, Park J (2019a) Biohydrogen generation from macroalgal biomass Chaetomorpha antennina through surfactant aided microwave disintegration. Front Energy Res 7:78. https://doi.org/10.3389/fenrg.2019.00078
Kumar G, Mathimani T, Rene ER, Pugazhendhi A (2019b) Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. Int J Hydrogen Energy 44:13106–13113. https://doi.org/10.1016/j.ijhydene.2019.03.131
Kumari D, Jain Y, Singh R (2021) A study on green pretreatment of rice straw using Petha wastewater and Mausami waste assisted with microwave for production of ethanol and methane. Energy Convers Manag X 10:100067. https://doi.org/10.1016/j.ecmx.2020.100067
Laurinavichene T, Tekucheva D, Laurinavichius K, Tsygankov A (2018) Enzyme and Microbial Technology Utilization of distillery wastewater for hydrogen production in one-stage and two-stage processes involving photofermentation. Enzyme Microb Technol 110:1–7. https://doi.org/10.1016/j.enzmictec.2017.11.009
Lee KS, Chen SL, Lin CY, Chang JS (2021) Converting waste molasses liquor into biohydrogen via dark fermentation using a continuous bioreactor. Int J Hydrogen Energy 46:16546–16554. https://doi.org/10.1016/j.ijhydene.2021.02.101
Lei M, Shen F, Hu J, Zhao L, Huang M, Zou J, Tian D, Yang G, Zeng Y, Deng S (2022) A novel way to facilely degrade organic pollutants with the tail-gas derived from PHP (phosphoric acid plus hydrogen peroxide) pretreatment of lignocellulose. J Hazard Mater 424:127517. https://doi.org/10.1016/j.jhazmat.2021.127517
Li N, Xiao X, Li C, Sheng X, Zhang J, Ping Q (2022a) Boosting VFAs production during the anaerobic acidification of lignocellulose waste pulp and paper mill excess sludge: ultrasonic pretreatment and inoculating rumen microorganisms. Ind Crops Prod 188:115613. https://doi.org/10.1016/j.indcrop.2022.115613
Li S, Zhao A, Chen Q, Cao Y, Xie Y, Wang J, Ao X (2022b) Effect of microwave pretreatment on catalytic gasification of spirit-based distillers’ grains to hydrogen-rich syngas. Waste Manag 149:239–247. https://doi.org/10.1016/j.wasman.2022.06.026
Li P, Yang C, Jiang Z, Jin Y, Wuet W (2023) Lignocellulose pretreatment by deep eutectic solvents and related technologies: a review. J Bioresour Bioprod 8:33–44. https://doi.org/10.1016/j.jobab.2022.11.004
Liang Z, Shen N, Lu C, Chen Y, Guanet Y (2021) Effective methane production from waste activated sludge in anaerobic digestion via formic acid pretreatment. Biomass Bioenergy 151:106176. https://doi.org/10.1016/j.biombioe.2021.106176
Lin R, Cheng J, Ding L, Song W, Liu M, Zhou J, Cen K (2016) Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using Enterobacter aerogenes. Bioresour Technol 207:213–219. https://doi.org/10.1016/j.biortech.2016.02.009
Liu Y, Zheng J, Xiao J, He X, Zhang K, Yuan S, Peng Z, Chen Z, Lin X (2019) Enhanced enzymatic hydrolysis and lignin extraction of wheat straw by triethylbenzyl ammonium chloride/lactic acid-based deep eutectic solvent pretreatment. ACS Omega 4:19829–19839. https://doi.org/10.1021/acsomega.9b02709
Liu H, Zhang Z, Lu C, Wang J, Wang K, Guo S, Zhang Q (2022) Effects of enzymatic hydrolysis and alkalization pretreatment on biohydrogen production by Chlorella photosynthesis. Bioresour Technol 349:126859. https://doi.org/10.1016/j.biortech.2022.126859
Lu H, Zhang L, Yan M, Wang K, Jiang J (2022) Screw extrusion pretreatment for high-yield lignocellulose nanofibrils (LCNF) production from wood biomass and non-wood biomass. Carbohydr Polym 277:118897. https://doi.org/10.1016/j.carbpol.2021.118897
Ma L, Cui Y, Cai R, Liu X, Zhang C, Xiao D (2015) Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob. Bioresour Technol 180:1–6. https://doi.org/10.1016/j.biortech.2014.12.078
Ma H, Lin Y, Jin Y, Gao M, Li H, Wang Q, Ge S, Cai L, Huang Z, Le QV, Xia C (2021) Effect of ultrasonic pretreatment on chain elongation of saccharified residue from food waste by anaerobic fermentation. Environ Pollut 268:115936. https://doi.org/10.1016/j.envpol.2020.115936
Mahmoodi-Eshkaftaki M, Ghani A (2022) An efficient process for improvement of biohydrogen and biomethane production from tomato waste: inhibitory effects of ultrasonic pretreatment. Fuel 328:125273. https://doi.org/10.1016/j.fuel.2022.125273
Manuel C, Carlos Q, Carmen P, Marisela VL (2022) Fungal solid-state fermentation of food waste for biohydrogen production by dark fermentation. Int J Hydrogen Energy 47:30062–30073. https://doi.org/10.1016/j.ijhydene.2022.06.313
Martín MI, García-Díaz I, López FA (2023) Properties and perspective of using deep eutectic solvents for hydrometallurgy metal recovery. Miner Eng 203:108306. https://doi.org/10.1016/j.mineng.2023.108306
Mehrez I, Chandrasekhar K, Kim W, Kim SH, Kumar G (2022) Comparison of alkali and ionic liquid pretreatment methods on the biochemical methane potential of date palm waste biomass. Bioresour Technol 360:127505. https://doi.org/10.1016/j.biortech.2022.127505
Micolucci F, Gottardo M, Bolzonella D, Pavan P, Majone M, Valentino F (2020) Pilot-scale multi-purposes approach for volatile fatty acid production, hydrogen and methane from an automatic controlled anaerobic process. J Clean Prod 277:124297. https://doi.org/10.1016/j.jclepro.2020.124297
Mishra P, Thakur S, Singh L, Wahid ZA, Sakinah M (2016) Enhanced hydrogen production from palm oil mill effluent using two stage sequential dark and photo fermentation. Int J Hydrogen Energy 41:18431–18440. https://doi.org/10.1016/j.ijhydene.2016.07.138
Mishra V, Jana AK, Maiti M, Gupta A (2017) Enhancement in multiple lignolytic enzymes production for optimized lignin degradation and selectivity in fungal pretreatment of sweet sorghum bagasse. Bioresour Technol 236:49–59. https://doi.org/10.1016/j.biortech.2017.03.148
Mishra P, Thakur S, Mahapatra DM, Wahid ZA, Liu H, Singh L (2018) Impacts of nano-metal oxides on hydrogen production in anaerobic digestion of palm oil mill effluent – a novel approach. Int J Hydrogen Energy 43:2666–2676. https://doi.org/10.1016/j.ijhydene.2017.12.108
Mlaik N, Khoufi S, Hamza M, Masmoudia MA, Sayadi S (2019) Enzymatic pre-hydrolysis of organic fraction of municipal solid waste to enhance anaerobic digestion. Biomass Bioenergy 127:105286. https://doi.org/10.1016/j.biombioe.2019.105286
Moia IC, Kanaropoulou A, Ghanotakis DF, Carlozzi P, Touloupakis E (2024) Photofermentative hydrogen production by immobilized Rhodopseudomonas sp. S16-VOGS3 cells in photobioreactors. Energy Rev 3:100055. https://doi.org/10.1016/j.enrev.2023.100055
Moreno-Andrade I, Carrillo-Reyes J, Santiago SG, Bujanos-Adame MC (2015) Biohydrogen from food waste in a discontinuous process: effect of HRT and microbial community analysis. Int J Hydrogen Energy 40:17246–17252. https://doi.org/10.1016/j.ijhydene.2015.04.084
Mozhiarasi V, Weichgrebe D, Srinivasan SV (2020) Enhancement of methane production from vegetable, fruit and flower market wastes using extrusion as pretreatment and kinetic modeling. Water Air Soil Pollut 231:126. https://doi.org/10.1007/s11270-020-04469-2
Mu H, Sun Q, Zhong M, Zhao C, Zhang Y, Wang L (2023) Significant enhancement of biomethane recovery in up-flow anaerobic sludge blanket reactor treating starch wastewater through oyster shells conditioning. J Clean Prod 432:139801. https://doi.org/10.1016/j.jclepro.2023.139801
Müller LJ, Kätelhön A, Bringezu S, McCoyd S, Suhe S, Edwardsf R, Sickg V, Kaiserc S, Cuéllar-Francah R, Khamlichii AE, Leej JH, Assena NVD, Bardow A (2020) The carbon footprint of the carbon feedstock CO2. Energy Environ Sci 13:2979–2992. https://doi.org/10.1039/d0ee01530j
Namdarimonfared M, Zilouei H, Tondro H (2023) Biological hydrogen production from paper mill effluent via dark fermentation in a packed bed biofilm reactor. Fuel 338:127231. https://doi.org/10.1016/j.fuel.2022.127231
Nanda S, Berruti F (2021a) Municipal solid waste management and landfilling technologies: a review. Environ Chem Lett 19:1433–1456. https://doi.org/10.1007/s10311-020-01100-y
Nanda S, Berruti F (2021b) Thermochemical conversion of plastic waste to fuels: a review. Environ Chem Lett 19:123–148. https://doi.org/10.1007/s10311-020-01094-7
Nanda S, Mohammad J, Reddy SN, Kozinski JA, Dalai AK (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers Bioref 4:157–191. https://doi.org/10.1007/s13399-013-0097-z
Nanda S, Rana R, Zheng Y, Kozinski JA, Dalai AK (2017) Insights on pathways for hydrogen generation from ethanol. Sustain Energy Fuels 1:1232–1245. https://doi.org/10.1039/C7SE00212B
Nanda S, Patra BR, Patel R, Bakos J, Dalai AK (2022) Innovations in applications and prospects of bioplastics and biopolymers: a review. Environ Chem Lett 20:379–395. https://doi.org/10.1007/s10311-021-01334-4
Nanda S, Pattnaik P, Patra BR, Kang K, Dalai AK (2023) A review of liquid and gaseous biofuels from advanced microbial fermentation processes. Fermentation 9:813. https://doi.org/10.3390/fermentation9090813
Nefise R, Kucukkara I, Murat A (2020) Hydrogen generation by electrolysis under subcritical water condition and the effect of aluminium anode. Int J Hydrogen Energy 45:12641–12652. https://doi.org/10.1016/j.ijhydene.2020.02.223
Newborough M, Cooley G (2020) Developments in the global hydrogen market: electrolyser deployment rationale and renewable hydrogen strategies and policies. Fuel Cells Bull 2020:16–22. https://doi.org/10.1016/S1464-2859(20)30486-7
Nguyen DT, Nguyen VH, Nanda S, Vo DVN, Nguyen VH, Tran TV, Nong LX, Nguyen TT, Bach LG, Abdullah B, Hong SS, Nguyen TV (2020) BiVO4 photocatalysis design and applications to oxygen production and degradation of organic compounds: a review. Environ Chem Lett 18:1779–1801. https://doi.org/10.1007/s10311-020-01039-0
Niño-Navarro C, Chairez I, Christen P, Canul-Chan M, García-Peña EI (2020) Enhanced hydrogen production by a sequential dark and photo fermentation process: effects of initial feedstock composition, dilution and microbial population. Renew Energy 147:924–936. https://doi.org/10.1016/j.renene.2019.09.024
Nirmala N, Praveen G, Amitkumar S, Rajan PSS, Baskaran A, Priyadharsini P, Kumar SPS, Dawn SS, Pavithra KG, Arun J, Pugazhendhi A (2023) A review on biological biohydrogen production: outlook on genetic strain enhancements, reactor model and techno-economics analysis. Sci Total Environ 896:165143. https://doi.org/10.1016/j.scitotenv.2023.165143
Niu Q, Kobayashi T, Takemura Y, Kubota K, Li YY (2015) Evaluation of functional microbial community’s difference in full-scale and lab-scale anaerobic digesters feeding with different organic solid waste: effects of substrate and operation factors. Bioresour Technol 193:110–118. https://doi.org/10.1016/j.biortech.2015.05.107
Nordin N, Illias R, Hasmaliana N, Ramli ANM, Selvasembian R, Azelee NIW, Rajagopal R, Thirupathi A, Chang SW, Ravindran B (2022) Highly sustainable cascade pretreatment of low-pressure steam heating and organic acid on pineapple waste biomass for efficient delignification. Fuel 321:124061. https://doi.org/10.1016/j.fuel.2022.124061
Nualsri C, Kongjan P, Reungsang A (2016) Direct integration of CSTR-UASB reactors for two-stage hydrogen and methane production from sugarcane syrup. Int J Hydrogen Energy 41:17884–17895. https://doi.org/10.1016/j.ijhydene.2016.07.135
Okolie JA, Nanda S, Dalai AK, Berruti F, Kozinski JA (2020) A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew Sustain Energy Rev 119:109546. https://doi.org/10.1016/j.rser.2019.109546
Okolie JA, Patra BR, Mukherjee A, Nanda S, Dalai AK, Kozinski JA (2021) Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy. Int J Hydrogen Energy 46:8885–8905. https://doi.org/10.1016/j.ijhydene.2021.01.014
Ortigueira J, Martins L, Pacheco M, Silva C, Moura P (2019) Improving the non-sterile food waste bioconversion to hydrogen by microwave pretreatment and bioaugmentation with Clostridium butyricum. Waste Manag 88:226–235. https://doi.org/10.1016/j.wasman.2019.03.021
Osman AI, Mehta N, Elgarahy AM, Hefny M, Al-Hinai A, Al-Muhtaseb AH, Rooney DW (2022) Hydrogen production, storage, utilisation and environmental impacts: a review. Environ Chem Lett 21:2639–2705. https://doi.org/10.1007/s10311-023-01613-2
Osman AI, Elgarahy AM, Eltaweil AS, Abd El-Monaem EM, El-Aqapa HG, Park Y, Hwang Y, Ayati A, Farghali M, Ihara I (2023a) Biofuel production, hydrogen production and water remediation by photocatalysis, biocatalysis and electrocatalysis. Environ Chem Lett 21:1315–1379. https://doi.org/10.1007/s10311-023-01581-7
Osman AI, Lai ZY, Farghali M, Yiin CL, Elgarahy AM, Hammad A, Ihara I, Al-Fatesh AS, Rooney DW, Yap PS (2023b) Optimizing biomass pathways to bioenergy and biochar application in electricity generation, biodiesel production, and biohydrogen production. Springer International Publishing
Our World in Data. Energy mix. https://ourworldindata.org/energy-mix. Accessed 26 Jan 2024
Palomo-Briones R, Razo-Flores E, Bernet N, Trably E (2017) Dark-fermentative biohydrogen pathways and microbial networks in continuous stirred tank reactors: novel insights on their control. Appl Energy 198:77–87. https://doi.org/10.1016/j.apenergy.2017.04.051
Pandey A, Sinha P, Pandey A (2020) ScienceDirect Hydrogen production by sequential dark and photofermentation using wet biomass hydrolysate of Spirulina platensis: response surface methodological approach. Int J Hydrogen Energy 46:7137–7146. https://doi.org/10.1016/j.ijhydene.2020.11.205
Patra BR, Mukherjee A, Nanda S, Dalai AK (2021a) Biochar production, activation and adsorptive applications: a review. Environ Chem Lett 19:2237–2259. https://doi.org/10.1007/s10311-020-01165-9
Patra BR, Nanda S, Dalai AK, Meda V (2021b) Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere 285:131431. https://doi.org/10.1016/j.chemosphere.2021.131431
Pattnaik F, Tripathi S, Patra BR, Nanda S, Kumar V, Dalai AK, Naik S (2021) Catalytic conversion of lignocellulosic polysaccharides to commodity biochemicals: a review. Environ Chem Lett 19:4119–4136. https://doi.org/10.1007/s10311-021-01284-x
Pattnaik F, Patra BR, Nanda S, Mohanty MK, Dalai AK, Rawat J (2024) Drivers and barriers in the production and utilization of second-generation bioethanol in India. Recycling 9:19. https://doi.org/10.3390/recycling9010019
Pérez-Pimienta JA, García-López RM, Méndez-Acosta HO, González-Álvarez V, Simmons BA, Méndoza-Pérez JA, Arreola-Vargas J (2021) Ionic liquid-water mixtures enhance pretreatment and anaerobic digestion of agave bagasse. Ind Crops Prod 171:113924. https://doi.org/10.1016/j.indcrop.2021.113924
Policastro G, Luongo V, Fabbricino M (2020) Biohydrogen and poly-β-hydroxybutyrate production by winery wastewater photofermentation: effect of substrate concentration and nitrogen source. J Environ Manag 271:111006. https://doi.org/10.1016/j.jenvman.2020.111006
Pour N, Webley PA, Cook PJ (2018) Potential for using municipal solid waste as a resource for bioenergy with carbon capture and storage (BECCS). Int J Greenhouse Gas Control 68:1–15. https://doi.org/10.1016/j.ijggc.2017.11.007
Pugazhendi A, Jamal MT, Banu R (2023) Biohydrogen production through energy efficient surfactant induced microwave pretreatment of macroalgae Ulva reticulata. Environ Res 236:116709. https://doi.org/10.1016/j.envres.2023.116709
Qi N, Zhao X, Zhang L, Gao M, Yu N, Liu Y (2021) Performance assessment on anaerobic co-digestion of Cannabis ruderalis and blackwater: ultrasonic pretreatment and kinetic analysis. Resour Conserv Recycl 169:105506. https://doi.org/10.1016/j.resconrec.2021.105506
Rahmani AM, Tyagi VK, Gunjyal N, Kazmi AA, Ojha CSP, Moustakas K (2023) Hydrothermal and thermal-alkali pretreatments of wheat straw: co-digestion, substrate solubilization, biogas yield and kinetic study. Environ Res 216:114436. https://doi.org/10.1016/j.envres.2022.114436
Rambabu K, Bharath G, Thanigaivelan A, Das DB, Show PL, Banat F (2021) Augmented biohydrogen production from rice mill wastewater through nano-metal oxides assisted dark fermentation. Bioresour Technol 319:124243. https://doi.org/10.1016/j.biortech.2020.124243
Ramos LR, Silva EL, Luis RW (2020) Thermophilic hydrogen and methane production from sugarcane stillage in two-stage anaerobic fluidized bed reactors. Int J Hydrogen Energy 45:5239–5251. https://doi.org/10.1016/j.ijhydene.2019.05.025
Rawoof SAA, Kumar PS, Vo DVN, Subramanian S (2021) Sequential production of hydrogen and methane by anaerobic digestion of organic wastes: a review. Environ Chem Lett 19:1043–1063. https://doi.org/10.1007/s10311-020-01122-6
Reddy K, Nasr M, Kumari S, Kumar S, Gupta SK, Enitan AM, Bux F (2017) Biohydrogen production from sugarcane bagasse hydrolysate: effects of pH, S/X, Fe2+, and magnetite nanoparticles. Environ Sci Pollut Res 24:8790–8804. https://doi.org/10.1007/s11356-017-8560-1
Regueira-marcos L (2023) Elucidating the role of pH and total solids content in the co-production of biohydrogen and carboxylic acids from food waste via lactate-driven dark fermentation. Fuel 338:1277238. https://doi.org/10.1016/j.fuel.2022.127238
Roebuck P, Kennedy K, Delatolla R (2019) Ultrasonic pretreatment for anaerobic digestion of suspended and attached growth sludges. Water Qual Res J 54:265–277. https://doi.org/10.2166/wqrj.2019.039
Ruggeri B, Malave ACL, Bernardi M, Fino D (2013) Energy efficacy used to score organic refuse pretreatment processes for hydrogen anaerobic production. Waste Manag 33:2225–2233. https://doi.org/10.1016/j.wasman.2013.06.024
Saba B, Bharathidasan AK, Ezeji TC, Cornish K (2023) Characterization and potential valorization of industrial food processing wastes. Sci Total Environ 868:161550. https://doi.org/10.1016/j.scitotenv.2023.161550
Safari M, Tondro H, Zilouei H (2022) Biohydrogen production from diluted-acid hydrolysate of rice straw in a continuous anaerobic packed bed biofilm reactor. Int J Hydrogen Energy 47:5879–5890. https://doi.org/10.1016/j.ijhydene.2021.11.222
Sahil S, Karvembu P, Kaur R, Katyal P, Phutela UG (2023) Enhanced biogas production from rice straw through pretreatment with cellulase producing microbial consortium. Energy Nexus 12:100246. https://doi.org/10.1016/j.nexus.2023.100246
Saidi M, Gohari MH, Ramezani AT (2020) Hydrogen production from waste gasification followed by membrane filtration: a review. Environ Chem Lett 18:1529–1556. https://doi.org/10.1007/s10311-020-01030-9
Sakinah N, Mohamed P, Wu S (2017) Influence of iron (II) oxide nanoparticle on biohydrogen production in thermophilic mixed fermentation. Int J Hydrogen Energy 42:27482–27493. https://doi.org/10.1016/j.ijhydene.2017.05.224
Sali A, Zeli B, Nigam KDP, Ti M (2023) Synergism of ionic liquids and lipases for lignocellulosic biomass valorization. Chem Eng J 461:142011. https://doi.org/10.1016/j.cej.2023.142011
Sanchez A, Ayala OR, Hernandez-Sanchez P, Valdez-Vazquez I, de León-Rodríguez A (2020) An environment-economic analysis of hydrogen production using advanced biorefineries and its comparison with conventional technologies. Int J Hydrogen Energy 45:27994–28006. https://doi.org/10.1016/j.ijhydene.2020.07.135
Santiago SG, Trably E, Latrille E, Buitrón G, Moreno-Andrade I (2019) The hydraulic retention time influences the abundance of Enterobacter, Clostridium and Lactobacillus during the hydrogen production from food waste. Lett Appl Microbiol 69:138–147. https://doi.org/10.1111/lam.13191
Santiago SG, Morgan-Sagastume JM, Monroy O, Moreno-Andrade I (2020) Biohydrogen production from organic solid waste in a sequencing batch reactor: an optimization of the hydraulic and solids retention time. Int J Hydrogen Energy 45:25681–25688. https://doi.org/10.1016/j.ijhydene.2019.11.224
Santos SC, Rosa PRF, Sakamoto IK, Varesche MBA, Silva EL (2014) Organic loading rate impact on biohydrogen production and microbial communities at anaerobic fluidized thermophilic bed reactors treating sugarcane stillage. Bioresour Technol 159:55–63. https://doi.org/10.1016/j.biortech.2014.02.051
Sarangi PK, Nanda S (2020) Biohydrogen production through dark fermentation. Chem Eng Technol 43:601–612. https://doi.org/10.1002/ceat.201900452
Saraphirom P, Reungsang A (2011) Biological hydrogen production from sweet sorghum syrup by mixed cultures using an anaerobic sequencing batch reactor (ASBR). Int J Hydrogen Energy 36:8765–8773. https://doi.org/10.1016/j.ijhydene.2010.08.058
Sarker TR, Nanda S, Meda V, Dalai AK (2023) Densification of waste biomass for manufacturing solid biofuel pellets: a review. Environ Chem Lett 21:231–264. https://doi.org/10.1007/s10311-022-01510-0c
Seifert K, Zagrodnik R, Stodolny M, Łaniecki M (2018) Biohydrogen production from chewing gum manufacturing residue in a two-step process of dark fermentation and photofermentation. Renew Energy 122:526–532. https://doi.org/10.1016/j.renene.2018.01.105
Senan S, Madihah A, Ping J, Jahim JM, Abdul PM, Mastar MS, Anuar N, Yunus MFM, Asis AJ, Wu SY (2019) Operation performance of up- flow anaerobic sludge blanket (UASB) bioreactor for biohydrogen production by self-granulated sludge using pre-treated palm oil mill effluent (POME) as carbon source. Renew Energy 134:1262–1272. https://doi.org/10.1016/j.renene.2018.09.062
Shanmugam SR, Lalman JA, Chaganti SR, Heath DD, Lau PCK, Shewa WA (2016) Long term impact of stressing agents on fermentative hydrogen production: effect on the hydrogenase flux and population diversity. Renew Energy 88:483–493. https://doi.org/10.1016/j.renene.2015.11.062
Sharma P, Melkania U (2017) Biochar-enhanced hydrogen production from organic fraction of municipal solid waste using co-culture of Enterobacter aerogenes and E. coli. Int J Hydrogen Energy 42:18865–18874. https://doi.org/10.1016/j.ijhydene.2017.06.171
Shaterzadeh MJ, Ataei SA (2017) The effects of temperature, initial pH, and glucose concentration on biohydrogen production from Clostridium acetobutylicum. Energy Sour A Recover Util Environ Eff 39:1118–1123. https://doi.org/10.1080/15567036.2017.1297875
Show KY, Lee DJ, Tay JH, Lin CY, Chang JS (2012) Biohydrogen production: current perspectives and the way forward. Int J Hydrogen Energy 37:15616–15631. https://doi.org/10.1016/j.ijhydene.2012.04.109
Sillero L, Solera R (2023) Effect of temperature on biohydrogen and biomethane production using a biochemical potential test with different mixtures of sewage sludge, vinasse and poultry manure. J Clean Prod 382:135237. https://doi.org/10.1016/j.jclepro.2022.135237
Sim YB, Jung JH, Baik JH, Park JH, Kumar G, Banu JR, Kim SH (2021) Dynamic membrane bioreactor for high rate continuous biohydrogen production from algal biomass. Bioresour Technol 340:125562. https://doi.org/10.1016/j.biortech.2021.125562
Singh S, Kumar R, Setiabudi HD, Nanda S, Vo DVN (2018) Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: a state-of-the-art review. Appl Catal A Gen 559:57–74. https://doi.org/10.1016/j.apcata.2018.04.015
Singh R, Singh S, Kumar M (2020) Impact of n-butanol as an additive with eucalyptus biodiesel-diesel blends on the performance and emission parameters of the diesel engine. Fuel 277:118178. https://doi.org/10.1016/j.fuel.2020.118178
Singh A, Srivastava A, Saidulu D, Kumar A (2022a) Advancements of sequencing batch reactor for industrial wastewater treatment: major focus on modifications, critical operational parameters, and future perspectives. J Environ Manage 317:115305. https://doi.org/10.1016/j.jenvman.2022.115305
Singh R, Paritosh K, Pareek N, Vivekanand V (2022b) Integrated system of anaerobic digestion and pyrolysis for valorization of agricultural and food waste towards circular bioeconomy: review. Bioresour Technol 360:127596. https://doi.org/10.1016/j.biortech.2022.127596
Singh R, Kumar R, Kumar P, Sarangi PK, Kovalev AA, Vivekanand V (2023a) Effect of physical and thermal pretreatment of lignocellulosic biomass on biohydrogen production by thermochemical route: a critical review. Bioresour Technol 369:128458. https://doi.org/10.1016/j.biortech.2022.128458
Singh T, Sehgal A, Singh R, Sharma S, Pal DB, Tashkandi HM, Raddadi R, Harakeh S, Haque S, Srivastava M, Hassan AA, Srivastava N, Gupta VK (2023b) Algal biohydrogen production: impact of biodiversity and nanomaterials induction. Renew Sustain Energy Rev 183:113389. https://doi.org/10.1016/j.rser.2023.113389
Sivagurunathan P, Anburajan P, Kumar G, Kim SH (2016) Effect of hydraulic retention time (HRT) on biohydrogen production from galactose in an up-flow anaerobic sludge blanket reactor. Int J Hydrogen Energy 41:21670–21677. https://doi.org/10.1016/j.ijhydene.2016.06.047
Sivaramakrishnan R, Ramprakash B, Ramadoss G (2021) High potential of Rhizopus treated rice bran waste for the nutrient-free anaerobic fermentative biohydrogen production. Bioresour Technol 319:124193. https://doi.org/10.1016/j.biortech.2020.124193
Sriyod K, Reungsang A, Plangklang P (2021) One-step multi enzyme pretreatment and biohydrogen production from Chlorella sp. biomass. Int J Hydrogen Energy 46:39675–39687. https://doi.org/10.1016/j.ijhydene.2021.09.232
Su H, Cheng J, Zhou J, Song W, Cen K (2010) Hydrogen production from water hyacinth through dark- and photo-fermentation. Int J Hydrogen Energy 35:8929–8937. https://doi.org/10.1016/j.ijhydene.2010.06.035
Subramaniam S, Karunanandham K, Raja ASM, Uthandi S (2023) Delignification of the cotton stalk and ginning mill waste via EnZolv pretreatment and optimization of process parameters using response surface methodology (RSM). Bioresour Technol 387:129655. https://doi.org/10.1016/j.biortech.2023.129655
Sumardiono S, Matin HHA, Sulistianingtias I, Nugroho TY, Budiyono B (2023) Effect of physical and biological pretreatment on sugarcane bagasse waste-based biogas production. Mater Today Proc 87:41–44. https://doi.org/10.1016/j.matpr.2023.01.372
Sunyoto NMS, Zhu M, Zhang Z, Zhang D (2016) Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour Technol 219:29–36. https://doi.org/10.1016/j.biortech.2016.07.089
Suruagy MVT, Ross AB, Babatunde A (2023) Influence of microwave temperature and power on the biomethanation of food waste under mesophilic anaerobic conditions. J Environ Manage 341:117900. https://doi.org/10.1016/j.jenvman.2023.117900
Tadeu L, Sayuri L, Kiyuna LSM, Garcia ML, Zaiat M (2016) Operational strategies for long-term biohydrogen production from sugarcane stillage in a continuous acidogenic packed-bed reactor. Int J Hydrogen Energy 41:8132–8145. https://doi.org/10.1016/j.ijhydene.2015.10.143
Taherzadeh MJ (2020) Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew Energy 152:1140–1148. https://doi.org/10.1016/j.renene.2020.01.138
Tang ZY, Li L, Tang W, Shen JW, Yang QZ, Ma C, He YC (2023) Significantly enhanced enzymatic hydrolysis of waste rice hull through a novel surfactant-based deep eutectic solvent pretreatment. Bioresour Technol 381:129106. https://doi.org/10.1016/j.biortech.2023.129106
Ulbig P, Hoburg D (2002) Determination of the calorific value of natural gas by different methods. Thermochim Acta 382:27–35. https://doi.org/10.1016/S0040-6031(01)00732-8
Vadalà M, Kröll E, Küppers M, Lupascu DC, Brunstermann R (2023) Hydrogen production via dark fermentation by bacteria colonies on porous PDMS-scaffolds. Int J Hydrogen Energy 8:0–10. https://doi.org/10.1016/j.ijhydene.2023.03.285
Wang J, Yin Y (2019) Progress in microbiology for fermentative hydrogen production from organic wastes. Crit Rev Environ Sci Technol 49:825–865. https://doi.org/10.1080/10643389.2018.1487226
Wang Z, Wang T, Si B, Watson J, Zhang Y (2021) Accelerating anaerobic digestion for methane production: potential role of direct interspecies electron transfer. Renew Sustain Energy Rev 145:111069. https://doi.org/10.1016/j.rser.2021.111069
Wang H, Zhao B, Zhu F, Chen Q, Zhou T, Wang Y (2023) Study on the reduction of chlorine and heavy metals in municipal solid waste incineration fly ash by organic acid and microwave treatment and the variation of environmental risk of heavy metals. Sci Total Environ 870:161929. https://doi.org/10.1016/j.scitotenv.2023.161929
Wei S (2016) The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl Microbiol Biotechnol 100:9821–9836. https://doi.org/10.1007/s00253-016-7926-5
Wu H, Wang C, Chen P, He AY, Xing FX, Kong XP, Jiang M (2017) Effects of pH and ferrous iron on the coproduction of butanol and hydrogen by Clostridium beijerinckii IB4. Int J Hydrogen Energy 42:6547–6555. https://doi.org/10.1016/j.ijhydene.2017.02.094
Wu Y, Xia C, Cao J, Garalleh HAL, Garaleh M, Khouj M, Pugazhendhi A (2023) A review on current scenario of nanocatalysts in biofuel production and potential of organic and inorganic nanoparticles in biohydrogen production. Fuel 338:127216. https://doi.org/10.1016/j.fuel.2022.127216
Wzorek M (2021) Solar drying of granulated waste blends for dry biofuel production. Environ Sci Pollut Res 28:34290–34299. https://doi.org/10.1007/s11356-021-12848-3
Xu J, Xu J, Zhang S, Xia J, Liu X, Chu X, Duan J, Li X (2017a) Synergistic effects of metal salt and ionic liquid on the pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.10.018
Xu Y, Lu Y, Dai X, Dong B (2017b) The influence of organic-binding metals on the biogas conversion of sewage sludge. Water Res 126:329–341. https://doi.org/10.1016/j.watres.2017.09.046
Xu Y, Sun S, Zhang C, Ma CY, Wen JL, Yuan TQ (2023) Complete valorization of bamboo biomass into multifunctional nanomaterials by reactive deep eutectic solvent pretreatment: towards a waste-free biorefinery. Chem Eng J 462:142213. https://doi.org/10.1016/j.cej.2023.142213
Yadav M, Singh A, Balan V, Pareek N, Vivekanand V (2019) Biological treatment of lignocellulosic biomass by Chaetomium globosporum: process derivation and improved biogas production. Int J Biol Macromol 128:176–183. https://doi.org/10.1016/j.ijbiomac.2019.01.118
Yahyazadeh A, Nanda S, Dalai AK (2024) A critical review of the sustainable production and application of methanol as a biochemical and bioenergy carrier. Reactions 5:1–19. https://doi.org/10.3390/reactions5010001
Yang G, Wang J (2018) Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles. Bioresour Technol 266:413–420. https://doi.org/10.1016/j.biortech.2018.07.004
Yang G, Wang J (2020) Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: energy conversion and microbial community. Energy Convers Manag 225:113436. https://doi.org/10.1016/j.enconman.2020.113436
Yang L, Xu F, Ge X, Li Y (2015) Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew Sustain Energy Rev 44:824–834. https://doi.org/10.1016/j.rser.2015.01.002
Yang X, Song Y, Ma S, Zhang X, Tan T (2020) Using γ-valerolactone and toluenesulfonic acid to extract lignin efficiently with a combined hydrolysis factor and structure characteristics analysis of lignin. Cellulose 27:3581–3590. https://doi.org/10.1007/s10570-020-03023-x
Yang Y, Liew RK, Tamothran AM, Foong SY, Yek PNY, Chia PW, Van Tran T, Peng W, Lam SS (2021) Gasification of refuse-derived fuel from municipal solid waste for energy production: a review. Environ Chem Lett 19:2127–2140. https://doi.org/10.1007/s10311-020-01177-5
Yang J, Wang W, Yang X, Long S, Tian X, Chen L, Liu X, Yang Q, Zhou T, Wang D (2023) Enhancing acidogenic fermentation of waste activated sludge via urea hydrogen peroxide pretreatment: performance and mechanisms. Bioresour Technol 386:129483. https://doi.org/10.1016/j.biortech.2023.129483
Yu Q, Qin L, Liu Y, Suna Y, Xua H, Wanga Z, Yuana Z (2019) In situ deep eutectic solvent pretreatment to improve lignin removal from garden wastes and enhance production of bio-methane and microbial lipids. Bioresour Technol 271:210–217. https://doi.org/10.1016/j.biortech.2018.09.056
Yu D, Xue Z, Mu T (2021) Eutectics: formation, properties, and applications. Chem Soc Rev 50:8596–8638. https://doi.org/10.1039/d1cs00404b
Yukesh KR, Kavitha S, Sivashanmugam P, Kumar G, Banu JR (2021) Ultrasonic induced mechanoacoustic effect on delignification of rice straw for cost effective biopretreatment and biomethane recovery. Sustain Energy Fuels 5:1832–1844. https://doi.org/10.1039/d0se01814g
Zhang X, Wang Z, Wu Z, Lua F, Tonga J, Zangb L (2010) Formation of dynamic membrane in an anaerobic membrane bioreactor for municipal wastewater treatment. Chem Eng J 165:175–183. https://doi.org/10.1016/j.cej.2010.09.013
Zhang D, Yuwen L, Peng L (2013) Parameters affecting the performance of immobilized enzyme. J Chem 2013:946248. https://doi.org/10.1155/2013/946248
Zhang J, Li W, Yang J, Li Z, Zhang J, Zhao W, Zang L (2021a) Cobalt ferrate nanoparticles improved dark fermentation for hydrogen evolution. J Clean Prod 316:128275. https://doi.org/10.1016/j.jclepro.2021.128275
Zhang J, Zhao W, Yang J, Li Z, Zhang J, Zang L (2021b) Comparison of mesophilic and thermophilic dark fermentation with nickel ferrite nanoparticles supplementation for biohydrogen production. Bioresour Technol 329:124853. https://doi.org/10.1016/j.biortech.2021.124853
Zhang YT, Wei W, Wang C, Ni BJ (2022) Understanding and mitigating the distinctive stresses induced by diverse microplastics on anaerobic hydrogen-producing granular sludge. J Hazard Mater 440:129771. https://doi.org/10.1016/j.jhazmat.2022.129771
Zhang M, Yang Y, Mou H, Pan A, Su X, Chen J, Lin H, Sun F (2023a) Enhanced methane yield in anaerobic digestion of waste activated sludge by combined pretreatment with fungal mash and free nitrous acid. Bioresour Technol 385:129441. https://doi.org/10.1016/j.biortech.2023.129441
Zhang Y, Wang X, Zhu W, Zhao Y, Wang N, Gao M, Wang Q (2023b) Anaerobic fermentation of organic solid waste: recent updates in substrates, products, and the process with multiple products co-production. Environ Res 233:116444. https://doi.org/10.1016/j.envres.2023.116444
Zhang Y, Zhao W, Li S, Zhang X, Wang S (2023c) Unraveling the mechanism of increased synthesis of hydrogen from an anaerobic fermentation by zinc ferrate nanoparticles: mesophilic and thermophilic situations comparison. Bioresour Technol 387:129617. https://doi.org/10.1016/j.biortech.2023.129617
Zhang Z, Ai F, Li Y, Zhu S, Wu Q, Duan Z, Liu H, Qian L, Zhang Q, Zhang Y (2023d) Co-production process optimization and carbon footprint analysis of biohydrogen and biofertilizer from corncob by photo-fermentation. Bioresour Technol 375:128814. https://doi.org/10.1016/j.biortech.2023.128814
Zhu S, Zhang Y, Zhang Z, Ai F, Zhang H, Li Y, Wang Y, Zhang Q (2023) Ascorbic acid-mediated zero-valent iron enhanced hydrogen production potential of bean dregs and corn stover by photo fermentation. Bioresour Technol 374:128761. https://doi.org/10.1016/j.biortech.2023.128761
Zou X, Yang R, Zhou X, Cao G, Zhu R, Ouyang F (2020) Effects of mixed alkali-thermal pretreatment on anaerobic digestion performance of waste activated sludge. J Clean Prod 259:120940. https://doi.org/10.1016/j.jclepro.2020.120940
Funding
SN acknowledges the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Research Chairs (CRC) program. SS is thankful to the Department of Science and Technology and the Ministry of Science and Technology (Government of India) for the Junior Research Fellowship program. VV and AAK acknowledge the funding received from the Department of Science and Technology and the Ministry of Science and Technology (Government of India) within the framework of the Indo-Russian Project (DST/INT/RUS/RSF/P-62/2021) and by the Russian Science Foundation (Project number: 22-49-02002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None declared.
Research involving human participants and/or animals
No conflicts, informed consent, and human or animal rights applicable.
Consent for publication
All authors agree to publish this article in Environmental Chemistry Letters.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahil, S., Singh, R., Masakapalli, S.K. et al. Biomass pretreatment, bioprocessing and reactor design for biohydrogen production: a review. Environ Chem Lett (2024). https://doi.org/10.1007/s10311-024-01722-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10311-024-01722-6