Abstract
The recovery of pure water and valuable substances from wastewater is a major challenge in the context of the circular economy, requiring advanced separation methods. However, actual membrane separation techniques such as forward osmosis are limited by membrane fouling and selectivity. Here, we synthesized composite membranes by crosslinking polyvinyl alcohol hydrogel, using both glutaraldehyde and borax as crosslinking agents, on top of cellulose ester membranes. We tested these composite membranes on model and real wastewater. Results show that the composite membranes retain ammonium effectively, maintain surface electroneutrality, and exhibit remarkable resistance to fouling by organic and biological contaminants. This is explained by the high hydrophilicity of the membrane surface after application of a hydrogel layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atmospheric nitrogen undergoes nitrogen fixation, converting it into ammonia and other nitrogen-based compounds. When ammonia enters water bodies, it transforms into ammonium, which, when present in freshwater, can result in pollution and eutrophication. Various techniques have been devised to remove and recover ammonium from wastewater, and one such method is membrane separation (Yao et al. 2022). A specific membrane-based approach is forward osmosis, utilizing salinity gradients to facilitate water permeation without applied pressure (Li et al. 2024).
Cellulose ester membranes, like cellulose acetate and cellulose triacetate, find extensive use in forward osmosis applications. These membranes are electroneutral and can be employed for ammonium enrichment and recovery from wastewater. In contrast to negatively-charged polyamide-based membranes, neutrally-charged membranes can retain ammonium effectively (Yao et al. 2022). Commercial cellulose triacetate membranes, in particular, exhibit remarkable ammonium rejection, reaching 99.7% (Gonzales et al. 2023). Conversely, polyamide-based membranes require modification to enhance ammonium retention (Gonzales et al. 2022; Li et al. 2022). However, it is essential to note that cellulose ester membranes are not inherently resistant to fouling (Li et al. 2018). To address this, modifications involving the incorporation of antifouling, electroneutral materials can be employed to develop fouling-resistant membranes with high ammonium retention.
Hydrogels, three-dimensional, water-absorbent, crosslinked hydrophilic polymers, are well-suited for water purification (Wang et al. 2022; Yang et al. 2022). Particularly, membranes incorporating hydrogels exhibit exceptional resistance to fouling due to superhydrophilicity, effectively increasing the adsorption of water molecules on the membrane surface (Amiri et al. 2022; Hu et al. 2022). Superhydrophilic surfaces create water barriers that prevent foulant molecules from adhering to the membrane surface, reducing fouling issues (Desiriani et al. 2023).
Polyvinyl alcohol is an environmentally-friendly hydrogel material known for its high water solubility (Hermani et al. 2023). Its polyhydroxy groups have a strong affinity for water (Sharma et al. 2018). To enhance its stability in aqueous systems and provide excellent chemical tolerance and mechanical properties, polyvinyl alcohol typically undergoes crosslinking with another substance, such as glutaraldehyde (Park et al. 2018; Zeng et al. 2023). Another advantageous property of polyvinyl alcohol is its electroneutrality, beneficial for certain applications.
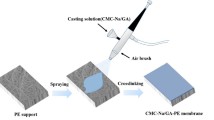
In this work, polyvinyl alcohol hydrogel composite membranes were developed for simultaneous wastewater treatment and resource recovery. Polyvinyl alcohol, previously known for improving membrane hydrophilicity, as well as the water permeability and fouling resistance (Razmgar and Nasiraee 2022), was utilized for its electroneutrality to effectively retain ammonium. A cellulose ester membrane was first prepared, followed by the application of polyvinyl alcohol crosslinking using two agents: glutaraldehyde and the food additive borax. The study assessed surface morphology and properties, intrinsic transport properties, osmotic performance, and resistance against model organic and biological foulants. Finally, these polyvinyl alcohol hydrogel composite membranes were employed for dewatering and concentrating real industrial wastewater. This research introduces a novel approach to preparing polyvinyl alcohol hydrogel composite membranes, employing a common food additive for the first time, according to the authors’ best knowledge. These membranes show promise for wastewater dewatering and ammonium enrichment.
Experimental
Membrane fabrication and characterization
Cellulose ester membranes were fabricated through nonsolvent-induced phase inversion of blended cellulose triacetate and cellulose acetate. Subsequently, a polyvinyl alcohol hydrogel layer was crosslinked onto these membranes using glutaraldehyde and borax. Various membrane characteristics, such as surface morphology, hydrophilicity, chemical properties, roughness, and zeta potential, were examined. The membranes’ intrinsic transport properties and osmotic performance were evaluated using laboratory-scale filtration systems (Figure S1), as detailed in literature (Gonzales et al. 2021). More detailed information on membrane fabrication, characterization, and performance assessment is available in the Supplementary Material.
Fouling
Static adsorption of lysozyme, bovine serum albumin, and E. coli was studied, following previous work (Wang et al. 2021). Dynamic fouling filtration experiments utilized a comprehensive solution containing various model organic foulants and E. coli solution as the feed solutions. The full details of these fouling experiments can be found in the Supplementary Material.
Ammonium enrichment from model and real wastewater
Long-term osmotic operations were conducted using model wastewater and locally-sourced real wastewater from Japan as feed solutions, with model seawater as the draw solution. The real wastewater was pre-filtered before use. Comprehensive information is available in the Supplementary Material.
Results and discussion
Membrane fabrication and characterization
The membranes’ intrinsic transport properties and characteristics underwent thorough evaluation. The membranes’ performance, including pure water permeability, solute permeability, and solute rejection, is detailed in the Supplementary Material (Figure S2). Figure 1a.1–3 depicts the altered surface morphology of the cellulose ester and polyvinyl alcohol hydrogel composite membranes, marked by a noticeable roughening and nodular structure due to the hydrogel layer. This change is supported by surface roughness measurements (Fig. 1b.1–3), indicating a rougher surface with the hydrogel coating compared to the pristine cellulose ester membrane. The cross-section morphology (Fig. 1c.1–3) shows no significant difference, except for the presence of a thin dense hydrogel layer atop the composite membranes. This hydrogel layer is further confirmed by energy dispersive X-ray spectroscopy (Fig. 1d.1–3), revealing thin layers with increased oxygen concentration, attributed to polyvinyl alcohol.
a Surface morphology, b surface roughness, c cross-section morphology, and d oxygen mapping of (1) pristine cellulose ester (CTA/CA) membrane, and polyvinyl alcohol hydrogel composite membranes crosslinked using (2) glutaraldehyde (CTA/CA-PVA-G) and (3) borax (CTA/CA-PVA-B); e Water contact angle and f zeta potential measurements of all membranes. The cellulose ester membrane (CTA/CA) is a cellulose triacetate and cellulose acetate (1:1) blend
To evaluate hydrophilicity, we conducted water contact angle measurements, as illustrated in Fig. 1e. The cellulose ester membrane displayed a water contact angle of 39.2°, while after the hydrogel layer coating, the values decreased to around 20°. Remarkably, both composite membranes achieved a water contact angle of 0° within 10 s, indicating superhydrophilicity of the hydrogel layer. Surface zeta potential measurements (Fig. 1f) revealed mostly electroneutral surfaces for both cellulose ester and glutaraldehyde-crosslinked composite membranes. The borax-crosslinked composite membrane exhibited a slightly negative charge at higher pH values, attributable to the tetraborate group of borax. Additional elemental composition characterization details are available in the Supplementary Material (Figure S3 and Table S1). Overall, the properties of the cellulose ester membranes were altered after polyvinyl alcohol coating. After evaluating the properties of the membranes, we assessed the membranes’ fouling resistance.
Fouling
We investigated the membranes’ susceptibility to fouling using various model foulants, as depicted in Fig. 2. Initially, static adsorption experiments were conducted with fluorescently-labeled proteins, negatively-charged bovine serum albumin and positively-charged lysozyme, with respective isoelectric points of 4.7 and 10.5 (Wang et al. 2021). These experiments, shown in Fig. 2a–b, revealed that both hydrogel composite membranes exhibited lower fluorescence intensities compared to the pristine cellulose ester membrane when exposed to these proteins. This indicates super resistance to foulant adhesion for the polyvinyl alcohol hydrogen composite membranes, regardless of foulant charge, despite having slightly higher membrane surface roughness. The enhanced performance is attributed to the superhydrophilicity of the hydrogel layer. Notably, the slightly negatively-charged borax-crosslinked membrane exhibited slightly higher lysozyme attachment than the glutaraldehyde-crosslinked membrane (Fig. 1f).
Confocal laser scanning microscope images of cellulose ester membrane (CTA/CA), glutaraldehyde-crosslinked hydrogel membrane (CTA/CA-PVA-G), and borax-crosslinked hydrogel membrane (CTA/CA-PVA-B) after incubation in a fluorescent bovine serum albumin, b fluorescent lysozyme, c E. coli, and d dynamic filtration using E. coli as feed solution; Water flux profiles of the membrane samples during dynamic filtration using e E. coli as feed solution and f foulant solution containing 500 mg/L of bovine serum albumin, sodium alginate, and humic acid as feed solution. After evaluating the membranes’ fouling resistance, the filtration performance was afterwards investigated
Visual representation in Fig. 2c.1–3 illustrates E. coli attachment after static adhesion experiments. The pristine membrane exhibited the highest bacterial adhesion, and the introduction of the hydrogel layer significantly increased membrane surface hydrophilicity, mitigating hydrophobic interactions and resulting in near-zero bacterial attachment on both hydrogel composite membranes. Dynamic biofouling filtration with E. coli further confirmed minimal bacterial adhesion on the hydrogel composite membranes (Fig. 2d.1–3). The flux profile during forward osmosis filtration Fig. 2e revealed that, although all membranes experienced flux decline during bacterial filtration, high flux recovery was observed for both hydrogel composite membranes, while the pristine membrane exhibited only around 50% flux of its initial flux.
We conducted dynamic filtration using a solution containing various model wastewater substances (Myat et al. 2014). Figure 2f shows that the pristine membrane experienced flux decline within the first 2 h, whereas the polyvinyl alcohol hydrogel composite membranes remained stable for up to 5 h. After backwashing, both hydrogel composite membranes achieved a flux recovery of around 95%, while the pristine membrane only recovered approximately 80% of its initial flux. Overall, the hydrogel composite membranes exhibited lower flux declines compared to the cellulose ester membrane by the end of the operation.
Ammonium enrichment from model and real wastewater
We conducted forward osmosis operations using model and real industrial wastewater as feed solutions. The osmotic performance with model wastewater, containing 100 mg/L NH4-N, is detailed in the Supplementary Material (Figure S4). Figure 3a illustrates an immediate flux decline for all membranes at the beginning of the osmotic process with real industrial wastewater. The pristine membrane experienced a sharp drop in flux, while the hydrogel composite membranes showed a less steep decline, indicating enhanced resistance to fouling by wastewater constituents. As the osmotic operation continued, flux declines occurred for all membranes, likely due to fouling or increased osmotic pressure of the feed solution during wastewater dewatering and concentration. Figure 3b compares the dewatering performance and final ammonium content of the concentrated wastewater. After 180 h, the pristine membrane removed 69% of water, resulting in a final ammonium content of 450 mg/L. In contrast, both polyvinyl alcohol hydrogel composite membranes exhibited superior dewatering, removing over 70% of water and resulting in final ammonium contents of over 675 mg/L.
a Water flux profile of the membranes during forward osmosis operation with real industrial wastewater (145 mg/L NH4-N content) as feed solution and 3.5% (w/w) NaCl as draw solution (Flow rate: 300 mL/min; Operation time: 180 h), and b Dewatering performance of each membrane and final NH4-N content in wastewater feed solution
We assessed the stability of the polyvinyl alcohol hydrogel layer through hydrophilicity and osmotic performance evaluation (Figure S5 and Table S2). Comparison with other forward osmosis membranes for ammonium retention in the literature are presented in Table S3. In conclusion, the comparable osmotic performance and exceptional fouling resistance of the hydrogel composite membranes suggest that this membrane preparation method is not only simple and scalable, but also effective for wastewater treatment applications.
Conclusion
We enhanced the fouling resistance of cellulose ester forward osmosis membranes by introducing a polyvinyl alcohol hydrogel coating, maintaining electroneutrality and ensuring effective ammonium retention. We employed conventional glutaraldehyde crosslinking and explored a one-step borax crosslinking approach. These hydrogel composite membranes displayed remarkable resistance to various foulants, effectively retaining ammonium, and exhibited exceptional performance in dewatering and concentrating real industrial wastewater. The use of the one-step borax crosslinking method for polyvinyl alcohol emerges as a straightforward and effective strategy for enhancing wastewater dewatering and ammonium concentration.
Availability of data and material
Data and material are available upon request.
Code availability
Not applicable.
References
Amiri S et al (2022) Polyvinyl alcohol and sodium alginate hydrogel coating with different crosslinking procedures on a PSf support for fabricating high-flux NF membranes. Chemosphere 308:136323. https://doi.org/10.1016/j.chemosphere.2022.136323
Desiriani R et al (2023) Control of organic and biological fouling of polyethersulfone membrane by blending and surface modification using natural additives. J Water Process Eng 55:104244. https://doi.org/10.1016/j.jwpe.2023.104244
Gonzales RR et al (2021) Facile development of comprehensively fouling-resistant reduced polyketone-based thin film composite forward osmosis membrane for treatment of oily wastewater. J Membr Sci 626:119185. https://doi.org/10.1016/j.memsci.2021.119185
Gonzales RR, Sasaki Y, Istirokhatun T, Li J, Matsuyama H (2022) Ammonium enrichment and recovery from synthetic and real industrial wastewater by amine-modified thin film composite forward osmosis membranes. Sep Purif Technol 297:121534. https://doi.org/10.1016/j.seppur.2022.121534
Gonzales RR et al (2023) Ammoniacal nitrogen concentration by osmotically assisted reverse osmosis. J Membr Sci 665:121122. https://doi.org/10.1016/j.memsci.2022.121122
Hermani M, Etemadi H, Khezraqa H (2023) Polycarbonate/polyvinyl alcohol thin film nanocomposite membrane incorporated with silver nanoparticles for water treatment. Braz J Chem Eng 40(3):863–872. https://doi.org/10.1007/s43153-022-00273-z
Hu D et al (2022) Anti-fouling nanofiltration membranes based on macromolecule crosslinked polyvinyl alcohol. J Ind Eng Chem 112:348–357. https://doi.org/10.1016/j.jiec.2022.05.032
Li J-Y et al (2018) Membrane fouling of forward osmosis in dewatering of soluble algal products: comparison of TFC and CTA membranes. J Membr Sci 552:213–221. https://doi.org/10.1016/j.memsci.2018.02.006
Li J et al (2022) Surface modification of thin film composite forward osmosis membrane using tris(2-aminoethyl)amine for enhanced ammonium recovery. Desalination 541:116002. https://doi.org/10.1016/j.desal.2022.116002
Li J et al (2024) Continuous purification of drugs by ionic liquid-drawn organic solvent forward osmosis and solute recovery. Environ Chem Lett 22(1):29–34. https://doi.org/10.1007/s10311-023-01641-y
Myat DT et al (2014) Experimental and computational investigations of the interactions between model organic compounds and subsequent membrane fouling. Water Res 48:108–118. https://doi.org/10.1016/j.watres.2013.09.020
Park MJ, Gonzales RR, Abdel-Wahab A, Phuntsho S, Shon HK (2018) Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 426:50–59. https://doi.org/10.1016/j.desal.2017.10.042
Razmgar K, Nasiraee M (2022) Polyvinyl alcohol-based membranes for filtration of aqueous solutions: a comprehensive review. Polym Eng Sci 62(1):25–43. https://doi.org/10.1002/pen.25846
Sharma G et al (2018) Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ Chem Lett 16(1):113–146. https://doi.org/10.1007/s10311-017-0671-x
Wang S-Y et al (2021) Surface charge control of poly(methyl methacrylate-co-dimethyl aminoethyl methacrylate)-based membrane for improved fouling resistance. Sep Purif Technol 279:119778. https://doi.org/10.1016/j.seppur.2021.119778
Wang X-T et al (2022) Biocompatible self-healing hydrogels based on boronic acid-functionalized polymer and laponite nanocomposite for water pollutant removal. Environ Chem Lett 20(1):81–90. https://doi.org/10.1007/s10311-021-01350-4
Yang Y et al (2022) Hydrogels for the removal of the methylene blue dye from wastewater: a review. Environ Chem Lett 20(4):2665–2685. https://doi.org/10.1007/s10311-022-01414-z
Yao X et al (2022) Surface modification of FO membrane for improving ammoniacal nitrogen (NH4+-N) rejection: investigating the factors influencing NH4+-N rejection. J Membr Sci 650:120429. https://doi.org/10.1016/j.memsci.2022.120429
Zeng H et al (2023) Gradient crosslinking optimization for the selective layer to prepare polyvinyl alcohol (PVA) nanofiltration (NF) membrane: the enhanced filtration performance and potential rejection for EDCs. J Membr Sci 675:121548. https://doi.org/10.1016/j.memsci.2023.121548
Acknowledgements
This work is part of a project (Project number: JPNP18016) commissioned by the New Energy and Industrial Technology Development Organization, Japan.
Funding
Open Access funding provided by Kobe University. New Energy and Industrial Technology Development Organization, Japan (Project number: JPNP18016).
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design; RRG, JL, PZ, XP, ZL, MH, ZM, KG: Material preparation, data collection, analysis; RRG: writing; RRG, HM: review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonzales, R.R., Li, J., Zhang, P. et al. Hydrogel membrane composite reduces fouling and retains ammonium efficiently. Environ Chem Lett 22, 1615–1621 (2024). https://doi.org/10.1007/s10311-024-01713-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-024-01713-7