Abstract
Humic substances play empirically several essential functions in biogeochemical cycles such as storage of carbon, pollutants, nutrients and water, yet the underlying mechanisms remain poorly known because their precise molecular structure is largely unknown so far. Here, we extracted humic substances from biomass waste of bell pepper, fennel, artichoke, coffee ground, coffee husks, and nursery residues. We analyzed humic extracts by ultra-high resolution Orbitrap Fusion Lumos Tribrid 1 M mass spectrometry, using both positive photoionization and negative electrospray ionization modes, and by 13C cross polarization/magic angle spinning nuclear magnetic resonance spectroscopy. We identified 5000–7000 unique organic compounds in humic substances by integrating photoionization with electrospray ionization. The chemical distribution of all components was depicted by nuclear magnetic resonance. Humic substances from green composts are composed by a wide variety of hydrophilic and hydrophobic moieties, thus providing the required biosurfactant properties for effective soil washing capacities, with carboxyl-rich alicyclic molecules, fatty acids, and phenolic acids as major constituents. Overall, our findings provide a major insight in the molecular structure of humic substances, thus opening research on mechanisms ruling the origin, fate and behavior of humic substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The utilization of recycled biomasses to generate bio-products is gaining momentum as it contributes to the advancement of the circular economy in numerous industries, including pharmaceuticals and agriculture (Barracosa et al. 2019; Duque-Acevedo et al. 2020). In particular, composting stands out as a notable biotechnology that uses a controlled aerobic microbial process to efficiently convert organic waste into sanitized and biochemically stable material (Ballardo et al. 2020; Monda et al. 2018). Significant quantities of residues and by-products are generated by horticultural crops and agro-industrial productions, which present valuable sources of organic matter. These resources can be effectively recycled through composting, resulting in the production of highly reactive humified material with versatile applications (Morales-Corts et al. 2018; Moretti et al. 2015; Verrillo et al. 2021a,b, 2022).
Apart from their conventional use as soil conditioners and fertilizers, composts have been found to contain valuable humic substances that can be extracted and used for remediating soils contaminated with organic pollutants (Conte et al. 2005; García-Díaz et al. 2015; Piccolo et al. 2021). Humic substances are organic colloidal components resulting from the physical, chemical, and microbiological transformation of plant and animal residues (Lichtfouse et al. 1998; Piccolo 2016; Dou et al. 2020). They are supramolecular associations of a wide diversity of heterogeneous molecules of relatively small mass held together by weak and dispersive forces (Piccolo 2002; Piccolo et al. 2019b). The intricate heterogeneity of humic substances grants them robust surfactant properties, which can be attributed to their composition of both hydrophobic and hydrophilic components, including fatty and phenolic acids. (Nebbioso and Piccolo 2011; Drosos et al. 2017; Vinci et al. 2022). With their abundant redox-active groups (i.e., carboxyl and hydroxyl functional groups), humic substances facilitate the formation of stable complexes with certain organic pollutants, metals, and radionuclides (Halim et al. 2003; Wang et al. 2023) and may serve as electron mediator during microbial respiration, promoting the biodegradation of organic pollutants and bio-reduction of heavy metals. As a result, humic substances also have the capability to effectively remove heavy metals from contaminated soils (Piccolo et al. 2019a, 2021; Bi et al. 2019). Indeed, when used alone or in combination with biomimetic catalysts, humic substances have demonstrated the ability to act as soil washing agents and facilitate the subsurface remediation of heavy metals and organic pollutants (Mao et al. 2015; Piccolo et al. 2019a; Tsang and Yip 2014; Yang and Hodson 2019).
A comprehensive understanding of the molecular characteristics of humic substances is necessary to fully exploit their chelating properties as biosurfactants. The lack of knowledge in this area can be attributed, in part, to the diverse molecular composition of humic substances obtained from various biomasses utilized in composting, as well as their varying levels of maturity. These factors play a substantial role in determining the effectiveness of humic substances in soil remediation procedures (Cozzolino et al. 2016; Pane et al. 2013; Piccolo et al. 2019a). A detailed characterization of humic substances samples extracted from various composted substrates is thus essential to elucidate the relationship between chemical composition and soil remediation activity. This characterization may help determine how different sources of compost affect the efficiency of humic substances in metal and radionuclides complexation and the removal of organic pollutants during soil washing (Piccolo et al. 2019a).
This study aims to probe the molecular composition of humic substances extracted from green composts made from residues of various agricultural biomasses (Fig. 1). Here, we used untargeted Orbitrap ultra-high-resolution mass spectrometry (UHRMS) analysis to measure with very high mass accuracy the mass-to-charge ratio of individual compounds forming the architecture of humic supra-structures, using both positive atmospheric pressure photoionization (APPI) and negative electrospray ionization (ESI) (Stenson et al. 2002; Marshall and Rodgers 2008; Nebbioso and Piccolo 2011; Maria et al. 2019; Solihat et al. 2019; Hawkes et al. 2020; Vinci et al. 2022). Additionally, we integrate UHRMS data with solid state 13C cross-polarization magic-angle-spinning nuclear magnetic resonance (13C-CPMAS-NMR) to provide a reliable description of the overall composition of carbon compounds in the complex humic matrices extracted from compost (Verrillo et al. 2021a,b, 2022). By combining these advanced analytical techniques, we aim to overcome the inherent limitations associated with individual approaches and gain a more accurate understanding of the molecular composition of humic substances.
Schematic representation of the workflow applied in this study. Organic composts were made with different mixes of organic waste. The humic substances extracted from the composts were analyzed by HRMS: high resolution mass spectrometry (Orbitrap Fusion Lumos Tribrid) and solid state 13C NMR: nuclear magnetic resonance spectroscopy (Bruker AV-300, equipped with a 4 mm MAS probe.)
Materials and methods
Green composts and humic substances extraction
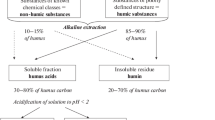
Green composts were produced at the composting center of the Experimental Farm of the University of Naples Federico II at Castel Volturno, Italy (Verrillo et al. 2021a,b). In this work, seven different composts were prepared by mixing residues of different agricultural biomasses (viz. lemon, bell pepper, fennels, artichokes, coffee ground, coffee husks, and nursery residues, Figure. S1A). Extraction of humic substances has been carried out according to the procedure reported by Verrillo et al. 2022 (Figure. S1B). The humic substances solutions were freeze-dried before analysis.
13C Cross-polarization magic-angle-spinning NMR spectroscopy
Solid-state 13C Cross-Polarization Magic-Angle-Spinning (CPMAS) NMR Spectroscopy spectra of humic substances were performed on a Bruker AV-300, equipped with a 4 mm MAS probe. The acquisition parameters were set as following: 10,000 Hz rotor spin rate; 2 s recycle time; 1H-power for CP 92, 16 W; 1H 90º pulse 3 µs; 13C power for CP 92, 75W; 1 ms of contact time; 40 ms of acquisition time; 4000 scans. Samples were packed in 4 mm zirconium rotors with Kel-F caps. To account for the molecular overlapping of solid state 13C NMR spectra, the signal resonances are conventionally grouped into six main spectral regions representative of the different types of carbon functional groups.
Ultrahigh-resolution orbitrap fusion lumos tribrid mass spectrometer
ESI (-) and APPI ( +) ionization modes were used to ionize a wider range of compounds in humic substances samples. The freeze-dried humic substances were dissolved and sonicated (~ 1 h) in a mixture of methanol and water 50:50 (v:v) and added with an aliquot of 20 µL of ammonia to reach pH 10 for negative electrospray ionization (-) measurements. In the case of APPI ( +) analyses, the extracted humic substances were instead dissolved and sonicated (~ 1 h) in a mixture of methanol:water:acetone 5:5:1 (v:v:v). Both ion sources (ESI and APPI) were interfaced to an Orbitrap Fusion Lumos Tribrid 1 M mass spectrometer (Thermo Scientific, San Jose, CA, USA). Detailed Orbitrap Fusion Lumos Tribrid1M mass spectrometer settings are reported in supplementary information (SI1). All peaks detected with a signal to noise ratio greater than 3 were processed using the Composer Software (Sierra Analytics, USA) and the relative detailed settings are discussed in supplementary information (SI2).
Results and discussions
Solid-state 13C CPMAS-NMR characterization of humic substances from different green composts
Our results revealed that humic substances isolated from the seven distinct compost samples exhibited a predominant presence of O-alkyl carbons (60–110 ppm). These O-alkylated carbons are associated with mono-, oligo-, and poly- saccharides (Table 1, Figure S2). Furthermore, within the humic substances analyzed, the alkyl-C chemical range constituted a maximum of 24.8% of the total area in the fennel-derived samples. This was followed by nursery residues, coffee husk, and artichoke, which accounted for approximately 24.1%, 23.6%, and 15.4% of the total C signals, respectively (Table 1). In general, a contrasting trend was observed for the distribution of total aromatic components, albeit with less pronounced variations (Table 1). Humic substances derived from artichoke, lemon, and nursery residues exhibited aryl and O-aryl-C components that accounted for 24.9, 24.2, and 23.4% of the total signal area, respectively (Table 1). In contrast, a relatively lower content of 20.1% was observed in humic substances derived from fennel. (Table 1).
The structural parameters calculated based on the relative abundance of various carbon groups (Table 1) can provide insights into the heterogeneous molecular characteristics of humic substances. These parameters can be utilized to establish correlations between the structural and conformational attributes of humic material and their bioactive properties, environmental reactivities, and metal binding capacity (Vaccaro et al. 2015; Savarese et al. 2022). We observed that the hydrophobic index values, which are close to unity for all the humic substance samples (Table 1) indicate a comparable contribution from polar/hydrophilic and apolar hydrophobic components. This finding supports the supramolecular pseudo-micellar conformation of humic materials in aqueous solutions, enabling them to effectively interact with both organic and inorganic contaminants (Mazzei and Piccolo 2015; Piccolo et al. 2021). Nonetheless, the O-alkyl ratio provides insights into the diversity of alkyl compounds with polar and apolar characteristics found in the humic extracts obtained from various green composts (Monda et al. 2018). The absence of NMR signals (Figure S2) around 84/89 ppm, which are typically associated with C4 carbons participating in glycosidic bonds, indicates a selective extraction of smaller carbohydrate components into the humic extracts from the green composts. Hence, our results suggest that the extracted humic substances primarily contain decomposed and fragmented carbohydrate compounds, rather than intact and rigid cellulose chains. The comparable aromatic index values, along with the overall decreasing trend in the Lignin ratio (Table 1), suggest the presence of polyphenolic and aromatic compounds derived from degraded lignin in the extracts. This finding aligns with previous studies by Savy et al. (2017).
Molecular chemodiversity of humic substances extracted from different composts
To our knowledge, this study represents the first instance of utilizing a combined approach involving negative electrospray ionization (-) and atmospheric pressure photoionization (APPI) ( +) Orbitrap UHRMS analysis for the examination of humic substances extracted from composts. By employing a combination of atmospheric pressure photoionization (APPI) in the positive ionization mode and negative electrospray ionization (−), we were able to amplify both the types and quantities of ions generated and detected. Our approach enabled a more comprehensive exploration of the compositional landscape within humic substances derived from composts, thereby facilitating a more detailed analysis. Hence, the simultaneous utilization of APPI ( +) and ESI (−) demonstrated significant disparities in the distribution and intensity of ions resolved within the mass range of 100 m/z to 800 m/z for ESI and 100 m/z to 1200 m/z for APPI. For example, Fig. 3A exhibits the full scan mass spectra obtained through negative electrospray ionization (ESI(-)) and atmospheric pressure photoionization (APPI ( +)) for the humic substances extracted from artichoke compost. In the ESI (−) spectrum, the highest intensity peaks were concentrated within the m/z 200–300 range. In contrast, the APPI ( +) analysis demonstrated a bimodal distribution, with the first local maximum centered around m/z 200 and a second maximum centered around m/z 350. Following data filtering (SI2), a substantial number of molecular formulae (N = 6356 individual formulae) were successfully assigned by integrating the datasets obtained from both negative electrospray ionization and positive atmospheric pressure photoionization. In total, there were 7413 distinct molecular formulae, with the highest molecular diversity observed in humic substances extracted from bell pepper composts. Conversely, we found that the lowest diversity was observed in humic substances derived from fennel composts, consisting of 5102 molecular formulae (Table S1).
Figure 2B displays the doughnut plots illustrating the relative abundance of different molecular series (compounds assigned as CcHhOoNnPpSs) detected in each ionization mode. In the ESI (−) spectrum of humic substances from the artichoke compost, approximately 44% of the identified molecular anions were CHO compounds, 43% were CHNO, 6% were CHNOS, and a small proportion consisted of phosphorous compounds (CHNOP and other P-containing compounds). Conversely, in the APPI ( +) analysis, 54% of the detected cations/radical species were CHNO compounds, followed by CHO (26%), CHN (8%), CH (3%), and CHOS (2.5%) in the humic substances from the artichoke compost. No phosphorous-containing compounds were detected (dataset provided in Table S2). All analyzed humic substances samples displayed a relatively high abundance of nitrogen-containing compounds (CHNO) and CHO compounds with oxygen content ranging from O1 to O8-O10 in nearly every sample (Fig. 2C). The prevalence of nitrogen-containing CHNO compounds over CHO compounds suggests the incorporation of nitrogen atoms into the macrostructure of humic substances, potentially facilitated by the inclusion of amino acids and protein residues within the supramolecular framework of humic matter (Piccolo et al. 2019b).
a Negative electrospray ionization [ESI (-) (red)] and atmospheric pressure photoionization [APPI ( +) (blue)] Fourier Transform—Orbitrap Fusion Lumos Tribrid 1 M full scan mass spectra of humic substances extracted from the artichoke compost; b doughnut plots featuring the relative abundance of each (color coded) molecular series detected in each ionization mode; c number of molecular formulae detected as a function of the number of oxygen atoms in both ESI (−) (top) and APPI ( +) (bottom) for each CHO, CHON, CHOS and CHNOS molecular series and d) summary of the relative abundances of compounds assigned to molecular families, combining both negative electrospray ESI (−) and positive photoionization APPI ( +). Molecular families containing C: carbon, H: hydrogen, O: oxygen, N: nitrogen, S: sulfur, P: phosphorus, Other: other compositions, CRAM: carboxyl-rich alicyclic molecules
The Van Krevelen diagram (Fig. 3) was employed to further analyze the detected compounds and their distribution among known biomolecular families, including lipid-like, protein-like, amino sugar-like, carbohydrate-like, condensed hydrocarbons, lignin-like, tannin-like, and aromatic structures (Gougeon et al. 2009; Roullier-Gall et al. 2014; Spano et al. 2021). In our APPI( +) analysis, all humic substances exhibited lower O/C and H/C ratios compared to the formulae analyzed using ESI(-) (Table S3). Additionally, higher aromaticity index and double bond equivalence (DBE) values were observed, consistent with previous research on humic substances extracted from soils (Vinci et al. 2022). Aromatic hydrocarbons generally have low H/C and O/C ratios, whereas aliphatic compounds exhibit high H/C and low O/C ratios. Hence, APPI ionization was more inclined to ionize less polar compounds with lower O/C ratios, increased aromaticity, higher N/C values, and a selective ionization of N-containing compounds. In contrast, negative electrospray ionization predominantly targeted more polar compounds with higher O/C and H/C ratios (Fig. 3).
Van Krevelen plot representing humic substances extracted from artichoke compost in both ionization techniques, negative electrospray ionization [ESI (−) left] and atmospheric pressure photoionization [APPI ( +) (right)]. Data color coded according to the molecular species CHO, CHON, CHOS and Others. Molecular families containing C: carbon, H: hydrogen, O: oxygen, N: nitrogen, S: sulfur, Other: other compositions. Dotted lines represent compound categorization based on aromaticity index (AI) and elemental H/C ratio. Zone α, condensed polycyclic aromatics (AI > 0.66): zone β, polyphenols (0.50 < AI < 0.66): zone χ, highly unsaturated and phenolic compounds (AI ≤ 0.50 and H/C < 1.5): and zone δ, aliphatic compounds (1.5 ≤ H/C ≤ 2.0)
The Van Krevelen diagrams (Fig. 3, Figure S3 and Table S1) demonstrate that the predominant components in the analyzed samples are lignin/CRAM molecules, accounting for 50% of the composition, followed by lipids and unsaturated hydrocarbons, each comprising 20% of the samples. The elemental ratios presented in Table S3 indicate that humic substances primarily consist of alicyclic and aromatic compounds characterized by high H/C ratios, low O/C ratios, low double bond equivalence (DBE), and AI indexes. These findings suggest the presence of carboxyl-rich alicyclic molecules (CRAM) within the humic substances, with carboxyl-C/aliphatic-C ratios ranging from 1:2 to 1:7. The presence of CRAM has been previously reported in dissolved organic matter (DOM) using NMR and ultra-high-resolution mass spectrometry (Hertkorn et al. 2013). CRAM compounds comprise highly carboxylated alicyclic structures that are associated with fused non-benzene rings, originating from both biotic and abiotic sources (Nebbioso et al. 2014; DiDonato and Hatcher 2017). Additionally, we observed abundant lipid compounds such as fatty acids, hydroxyacids, and alcohols, which result from the microbial degradation of biomasses during composting (Spaccini and Piccolo 2007). Furthermore, the elevated N/C ratio observed in coffee husk, coffee ground, and nursery residues indicates a higher concentration of nitrogen-containing compounds in the corresponding humic extracts.
Discussion on the molecular composition in relation to soil washing for remediation purposes
The molecular characterization of humic substances extracted from green compost for future soil washing experiments has not been previously explored. Previous studies have focused on examining humic substances derived from other sources, such as manure compost, solid waste, and sewage sludge, in order to assess their molecular composition (Kulikowska et al. 2015a, 2015b; Mao et al. 2015; Piccolo et al. 2019a; Tsang and Yip 2014; Yang and Hodson 2019). The hydrophobic and hydrophilic constituents of humic substances exhibit self-assembly properties, forming pseudo-micelles, which facilitate the redistribution of organo-metallic compounds. Additionally, the presence of carboxylic functional groups enhances the complexation and effective mobilization of organo-metals from soil environments (Ardakani and Stevenson 1972). The complexation abilities of humic molecules are influenced by their molecular composition, which is still not fully understood and can vary based on the source and type of compost (Monda et al. 2018). Hence, the effectiveness of humic substances in pollutant removal through soil washing may be influenced by the specific compost source employed. In this study, our objective was to examine the molecular composition of humic substances extracted from various types of green composts and compare them to those extracted from manure compost, which has previously shown successful soil washing results in contaminated sites (Piccolo et al. 2019a). Furthermore, the use of green compost offers several sustainability advantages over the application of humic substances derived from manure compost, solid waste, and sewage sludge. Green compost, comprising plant materials, significantly reduces environmental impact by minimizing methane and other potential greenhouse gas emissions. Additionally, it eliminates concerns associated with animal waste and its potential contribution to water pollution. The use of renewable and locally available resources in green compost production avoids the need for long-distance transportation. Moreover, carbon sequestration occurs through the capture and storage of atmospheric carbon dioxide into the soil, thereby assisting in mitigating climate change.
To accomplish this objective, we conducted comprehensive characterization using untargeted ultra-high-resolution mass spectrometry (UHRMS) in both atmospheric pressure photoionization (APPI) and negative electrospray ionization (ESI) modes, alongside 13C cross-polarization magic angle spinning nuclear magnetic resonance (13C-CPMAS-NMR) spectroscopy. Our findings revealed that humic substances extracted from green composts exhibited a notably high abundance of carboxyl-rich alicyclic molecules (CRAM). These CRAM compounds are recognized for their strong metal complexation properties, suggesting their potential effectiveness in soil washing for pollutant removal (Piccolo et al. 2019a). Through a comparison of the molecular composition of humic substances from green composts and manure composts (Piccolo et al. 2019a), we observed similarities in the content of carboxyl and aromatic carbon between the composts analyzed in this study.
Consequently, it can be anticipated that humic substances derived from green composts with higher levels of carboxyl and phenolic functional groups would exhibit superior abilities to complex heavy metals and aid in the separation of organo-metal complexes from contaminated soils. Our analysis further demonstrated the presence of carbohydrate components, particularly O-alkyl carbons associated with saccharides, in the humic substances from green composts. The relative proportions of alkyl and aromatic carbon components varied across different composts, indicating differences in the composition of humic substances. The coexistence of hydrophilic and hydrophobic constituents contributed to the formation of supramolecular pseudo-micellar structures within the humic substances.
Overall, our study provided valuable insights into the molecular composition of humic substances derived from diverse compost sources and their potential applicability in soil remediation. These findings underscore the significance of comprehending the compost materials and their influence on the efficacy of humic substances in pollutant removal from soils. Future research endeavors will focus on applying humic substances sourced from various green composts in soil washing experiments to determine the most effective remediation approach based on compost composition.
Conclusions
By employing advanced techniques such as ultra-high-resolution mass spectrometry (UHRMS) and nuclear magnetic resonance (NMR), this study unveiled the diverse molecular composition of humic substances extracted from different green composts derived from various agricultural biomasses. Our findings highlighted the presence of similar molecular components in these humic extracts, with a substantial proportion consisting of carboxyl-rich alicyclic molecules (CRAM). Alongside CRAM, fatty acids, and phenolic acids likely contribute to the stabilization of the supramolecular pseudo-micellar structure of humic substances, which, when in aqueous solutions, could potentially exhibit effective soil washing capabilities for the removal of both organic and inorganic contaminants. This research represents the first comprehensive characterization, to the best of our knowledge, of the molecular composition of humic substances derived from diverse green composts, employing high-resolution mass spectrometry with a mass error of less than 1 ppm, enabling the identification of 5000–7000 mass components. The significant presence of carboxyl-rich aliphatic and aromatic compounds, combined with their notable hydrophobicity, suggests their potential effectiveness as biosurfactants for soil washing applications. This detailed understanding of humus extracted from green composts represents a significant advancement in soil washing technologies, emphasizing the utilization of natural and sustainable recycled biomasses rather than non-renewable sources like peat, lignite, organic solvents, or synthetic surfactants, which can have detrimental effects on soil biological activity and biodiversity. In summary, green composting aligns with sustainable principles by harnessing renewable resources, reducing waste, promoting nutrient recycling, and supporting ecological balance in agriculture. The elucidation of the molecular composition of humic substances from green composts enhances our knowledge and paves the way for the development of soil washing techniques based on environmentally friendly and sustainable approaches.
Availability of data and material (data transparency)
Data are available upon request to the corresponding author.
Code availability
Not applicable.
References
Ardakani MS, Stevenson FJ (1972) A modified ion-exchange technique for the determination of stability constants of metal-soil organic matter complexes. American Society of Agronomy
Ballardo C, Vargas-García MC, del Sánchez A, Barrena R, Artola A (2020) Adding value to home compost: Biopesticide properties through Bacillus thuringiensis inoculation. Waste Manage 106:32–43. https://doi.org/10.1016/j.wasman.2020.03.003
Barracosa P, Barracosa M, Pires E (2019) Cardoon as a sustainable crop for biomass and bioactive compounds production. Chem Biodiversity
Bi D, Yuan G, Wei J, Xiao L, Feng L, Meng F, Wang J (2019) A soluble humic substance for the simultaneous removal of cadmium and arsenic from contaminated soils. Int J Environ Res Public Health 16(24):4999. https://doi.org/10.3390/ijerph16244999
Conte P, Agretto A, Spaccini R, Piccolo A (2005) Soil remediation: humic acids as natural surfactants in the washings of highly contaminated soils. Environ Pollut 135:515–522. https://doi.org/10.1016/j.envpol.2004.10.006
Cozzolino V, Di Meo V, Monda H, Spaccini R, Piccolo A (2016) The molecular characteristics of compost affect plant growth, arbuscular mycorrhizal fungi, and soil microbial community composition. Biol Fertil Soils 52:15–29. https://doi.org/10.1007/s00374-015-1046-8
DiDonato N, Hatcher PG (2017) Alicyclic carboxylic acids in soil humic acid as detected with ultrahigh resolution mass spectrometry and multi-dimensional NMR. Org Geochem 112:33–46. https://doi.org/10.1016/j.orggeochem.2017.06.010
Dou S, Shan J, Song X, Cao R, Wu M, Li C, Guan S (2020) Are humic substances soil microbial residues or unique synthesized compounds? a perspective on their distinctiveness. Pedosphere 30(2):159–167. https://doi.org/10.1016/S1002-0160(20)60001-7
Drosos M, Nebbioso A, Mazzei P, Vinci G, Spaccini R, Piccolo A (2017) A molecular zoom into soil Humeome by a direct sequential chemical fractionation of soil. Sci Total Environ 586:807–816. https://doi.org/10.1016/j.scitotenv.2017.02.059
Duque-Acevedo M, Belmonte-Ureña LJ, Cortés-García FJ, Camacho-Ferre F (2020) Agricultural waste: review of the evolution, approaches and perspectives on alternative uses. Global Ecol Conserv 22:e00902. https://doi.org/10.1016/j.gecco.2020.e00902
García-Díaz C, Nebbioso A, Piccolo A, Barrera-Cortés J, Martínez-Palou R (2015) Remediation of hydrocarbon-contaminated soil by washing with novel chemically modified humic substances. J Environ Qual 44:1764–1771. https://doi.org/10.2134/jeq2014.09.0399
Gougeon RD, Lucio M, Frommberger M, Peyron D, Chassagne D, Alexandre H, Feuillat F, Voilley A, Cayot P, Gebefügi I, Hertkorn N, Schmitt-Kopplin P (2009) The chemodiversity of wines can reveal a metabologeography expression of cooperage oak wood. Proc Natl Acad Sci USA 106:9174–9179. https://doi.org/10.1073/pnas.0901100106
Halim M, Conte P, Piccolo A (2003) Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances. Chemosphere 52:265–275. https://doi.org/10.1016/S0045-6535(03)00185-1
Hawkes JA, D’Andrilli J, Agar JN, Barrow MP, Berg SM, Catalán N, Chen H, Chu RK, Cole RB, Dittmar T, Gavard R, Gleixner G, Hatcher PG, He C, Hess NJ, Hutchins RHS, Ijaz A, Jones HE, Kew W, Khaksari M, Palacio Lozano DC, Lv J, Mazzoleni LR, Noriega-Ortega BE, Osterholz H, Radoman N, Remucal CK, Schmitt ND, Schum SK, Shi Q, Simon C, Singer G, Sleighter RL, Stubbins A, Thomas MJ, Tolic N, Zhang S, Zito P, Podgorski DC (2020) An international laboratory comparison of dissolved organic matter composition by high resolution mass spectrometry: Are we getting the same answer? Limnol Oceanogr Methods 18:235–258. https://doi.org/10.1002/lom3.10364
Hertkorn N, Harir M, Koch BP, Michalke B, Schmitt-Kopplin P (2013) High-Field NMR spectroscopy and FTICR mass spectrometry: powerful discovery tools for the molecular level characterization of marine dissolved organic matter. Biogeosciences 10:1583–1624
Kulikowska D, Gusiatin ZM, Bułkowska K, Kierklo K (2015a) Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 136:42–49. https://doi.org/10.1016/j.chemosphere.2015.03.083
Kulikowska D, Gusiatin ZM, Bułkowska K, Klik B (2015b) Feasibility of using humic substances from compost to remove heavy metals (Cd, Cu, Ni, Pb, Zn) from contaminated soil aged for different periods of time. J Hazard Mater 300:882–891. https://doi.org/10.1016/j.jhazmat.2015.08.022
Lichtfouse E, Chenu C, Baudin F, Leblond C, Da Silva M, Béhar F, Derenne S, Largeau C, Wehrung P, Albrecht P (1998) A novel pathway of soil organic matter formation by selective preservation of resistant straight-chain biopolymers: chemical and isotope evidence. Org Geochem 28(6):411–415. https://doi.org/10.1016/S0146-6380(98)00005-9
Mao X, Jiang R, Xiao W, Yu J (2015) Use of surfactants for the remediation of contaminated soils: a review. J Hazard Mater 285:419–435. https://doi.org/10.1016/j.jhazmat.2014.12.009
Maria E, Crançon P, Lespes G, Bridoux MC (2019) Spatial variation in the molecular composition of dissolved organic matter from the podzol soils of a temperate pine forest. ACS Earth Space Chem 3:1685–1696. https://doi.org/10.1021/acsearthumicsubstancespacechem.9b00164
Marshall AG, Rodgers RP (2008) Petroleomics: Chemistry of the underworld. Proc Natl Acad Sci USA 105:18090–18095. https://doi.org/10.1073/pnas.0805069105
Mazzei P, Piccolo A (2015) Interactions between natural organic matter and organic pollutants as revealed by NMR spectroscopy: interactions between natural organic matter and organic pollutants. Magn Reson Chem 53:667–678. https://doi.org/10.1002/mrc.4209
Monda H, Cozzolino V, Vinci G, Drosos M, Savy D, Piccolo A (2018) Molecular composition of the Humeome extracted from different green composts and their biostimulation on early growth of maize. Plant Soil 429:407–424. https://doi.org/10.1007/s11104-018-3642-5
Morales-Corts MR, Pérez-Sánchez R, Gómez-Sánchez MÁ (2018) Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soilborne pathogens. Sci Agric (Piracicaba, Braz.) 75:400–409. https://doi.org/10.1590/1678-992x-2016-0439
Moretti SML, Bertoncini EI, Abreu-Junior CH (2015) Composting sewage sludge with green waste from tree pruning. Sci Agric (piracicaba, Braz.) 72:432–439. https://doi.org/10.1590/0103-9016-2014-0341
Nebbioso A, Piccolo A (2011) Basis of a Humeomics science: chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromol 12:1187–1199. https://doi.org/10.1021/bm101488e
Nebbioso A, Piccolo A, Lamshoft M, Spiteller M (2014) Molecular characterization of an end-residue of humeomics applied to a soil humic acid. RSC Adv 4:23658–23665
Pane C, Piccolo A, Spaccini R, Celano G, Villecco D, Zaccardelli M (2013) Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl Soil Ecol 65:43–51. https://doi.org/10.1016/j.apsoil.2013.01.002
Piccolo A (2002) The Supramolecular structure of humic substances. A novel understanding of humus chemistry and implications in soil Science. Adv Agron 75:57–134. https://doi.org/10.1016/S0065-2113(02)75003-7
Piccolo, A., 2016. In memoriam Prof. F.J. Stevenson and the Question of humic substances in soil. Chem. Biol. Technol. Agric. 3, 23. https://doi.org/10.1186/s40538-016-0076-2
Piccolo A, Spaccini R, De Martino A, Scognamiglio F, di Meo V (2019a) Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 225:150–156. https://doi.org/10.1016/j.chemosphere.2019.03.019
Piccolo A, Spaccini R, Savy D, Drosos M, Cozzolino V (2019b) The soil humeome: chemical structure, functions and technological perspectives. In: Vaz S. jr. (ed) Sustainable agrochemistry: a compendium of technologies. Springer Nature, Heidelberg, pp 183–222. https://doi.org/10.1007/978-3-030-17891-8
Piccolo A, De Martino A, Scognamiglio F, Ricci R, Spaccini R (2021) Efficient simultaneous removal of heavy metals and polychlorobiphenyls from a polluted industrial site by washing the soil with natural humic surfactants. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-12484-x
Roullier-Gall C, Boutegrabet L, Gougeon RD, Schmitt-Kopplin P (2014) A grape and wine chemodiversity comparison of different appellations in Burgundy: vintage vs terroir effects. Food Chem 152:100–107. https://doi.org/10.1016/j.foodchem.2013.11.056
Savarese C, di Meo V, Cangemi S, Verrillo M, Savy D, Cozzolino V, Piccolo A (2022) Bioactivity of two different humic materials and their combination on plants growth as a function of their molecular properties. Plant Soil 472:509–526. https://doi.org/10.1007/s11104-021-05267-3
Savy D, Mazzei P, Drosos M, Cozzolino V, Lama L, Piccolo A (2017) Molecular characterization of extracts from biorefinery wastes and evaluation of their plant biostimulation. Sustainable Chemistry and Engineering 5:9023–9031. https://doi.org/10.1021/acssuschemeng.7b01928
Solihat NN, Acter T, Kim D, Plante AF, Kim S (2019) Analyzing solid-phase natural organic matter using laser desorption ionization ultrahigh resolution mass spectrometry. Anal Chem 91:951–957. https://doi.org/10.1021/acs.analchem.8b04032
Spaccini R, Piccolo A (2007) Molecular characterization of compost at increasing stages of maturity. 2. thermochemolysis-GC-MS and 13C-CPMAS-NMR spectroscopy. J Agric Food Chem 55:2303–2311
Spano M, Maccelli A, Di Matteo G, Ingallina C, Biava M, Crestoni ME, Bardaud J-X, Giusti AM, Mariano A, Scotto D’Abusco A, Sobolev AP, Lasalvia A, Fornarini S, Mannina L (2021) Metabolomic profiling of fresh goji (Lycium barbarum L.) berries from two cultivars grown in central italy: a multi-methodological approach. Molecules 26:5412. https://doi.org/10.3390/molecules26175412
Stenson AC, Landing WM, Marshall AG, Cooper WT (2002) Ionization and fragmentation of humic substances in electrospray ionization fourier transform-ion cyclotron resonance mass spectrometry. Anal Chem 74:4397–4409. https://doi.org/10.1021/ac020019f
Tsang DCW, Yip ACK (2014) Comparing chemical-enhanced washing and waste-based stabilisation approach for soil remediation. J Soils Sediments 14:936–947. https://doi.org/10.1007/s11368-013-0831-y
Vaccaro S, Ertani A, Nebbioso A, Muscolo A, Quaggiotti S, Piccolo A, Nardi S (2015) Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem Biol Technol Agric 2:5
Verrillo M, Cozzolino V, Spaccini R, Piccolo A (2021a) Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in Basil leaves. Chem Biol Technol Agric 8:28. https://doi.org/10.1186/s40538-021-00226-7
Verrillo M, Salzano M, Cozzolino V, Spaccini R, Piccolo A (2021b) Bioactivity and antimicrobial properties of chemically characterized compost teas from different green composts. Waste Manage 120:98–107. https://doi.org/10.1016/j.wasman.2020.11.013
Verrillo M, Salzano M, Savy D, Di Meo V, Valentini M, Cozzolino V, Piccolo A (2022) Antibacterial and antioxidant properties of humic substances from composted agricultural biomasses. Chem Biol Technol Agric 9:28. https://doi.org/10.1186/s40538-022-00291-6
Vinci G, Piccolo A, Bridoux M (2022) Complementary ESI and APPI high resolution mass spectrometry unravel the molecular complexity of a soil humeome. Anal Chim Acta 1194:339398. https://doi.org/10.1016/j.aca.2021.339398
Wang C, Cheng T, Zhang D, Pan X (2023) Electrochemical properties of humic acid and its novel applications: a tip of the iceberg. Sci. Tot. Environ. 863:160755. https://doi.org/10.1016/j.scitotenv.2022.160755
Yang T, Hodson ME (2019) Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci Total Environ 647:290–300. https://doi.org/10.1016/j.scitotenv.2018.07.457
Funding
Funding for this work was provided by the Commissariat à l’Energie Atomique et aux Energies Alternatives.
Author information
Authors and Affiliations
Contributions
AP, RS and MCB contributed to the design and implementation of the research. LMS, MCB, MV, SC, IM performed the experiments, analyzed, and elaborated the data. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethics approval (include appropriate approvals or waivers)
Not applicable.
Consent to participate
Not applicable.
Consent for publication (include appropriate statements)
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stancampiano, L.M., Verrillo, M., Cangemi, S. et al. The molecular composition of humic substances extracted from green composts and their potential for soil remediation. Environ Chem Lett 21, 2489–2498 (2023). https://doi.org/10.1007/s10311-023-01619-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01619-w