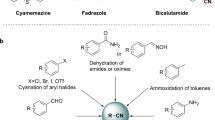
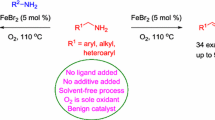
Abstract
Catalysis by first-row transition metals is of increasing interest in the context of the scarcity of chemical resources. For instance, iron is promising due to its abundance, low toxicity and unique electronic features. Here we synthesized quinazoline alkaloids from alkynoic acids and functionalized amines in the presence of iron dibromide and pyridine in toluene or, alternatively, in a solventless reaction system. We studied iron sources, reaction media and the effect of additives. Results show 39–99% yields and regioselective preparation of nitrogen- and oxygen-containing scaffolds. This is the first example of a cascade process involving alkynoic acids catalyzed by iron. Fe is more abundant, cheaper and less toxic than other Au, Cu and Ru catalysts previously reported for similar transformations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catalysis is one of the pillars of green chemistry. Indeed, one of the main strategies for the minimization of the amount of unwanted by-products is the replacement of stoichiometric chemical procedures by efficient catalytic protocols (Anastas and Warner 1998). Precious metals are prevalent in catalysis, but the decreasing availability of these inherently scarce metals has led to mining and refining of lower-grade metal ores, costly operations that require a large amount of resources and that cause soil erosion and pollution of groundwater, surface water and soil. On this basis, implementation of increasingly sustainable processes has latterly spurred the search for more available and less toxic metal catalysts (Ludwig and Schindler 2017).
Iron is one of the most abundant metals in earth and an essential element for many living organisms (Expert 2012; Crichton 2016). It has been known for long that iron can promote several organic transformations, but its catalytic role akin to that of some precious late transition metals remained unrevealed until recently. In addition to the wide range of oxidation states that iron can adopt (from − 2 to + 5), this convenient metal has the ability to transfer one or two electrons to a substrate, thus enabling not only radical reactions but also processes based on oxidative addition and reductive elimination steps (Rana et al. 2021; Casnati et al. 2020; DaBell and Thomas 2020; Zhang et al. 2020; Guđmundsson and Bäckvall 2020; Piontek et al. 2018; Wei and Darcel 2019; Bauer and Knölker 2015; Bolm et al. 2014). In this regard, the activation of carbon–carbon triple bond of alkynes by iron Lewis acids, or even low-valent iron complexes, can promote several annulation, cycloisomerization (enyne derivatives and allenols) and other cyclization processes leading to the formation of several heterocycles such as benzo- and dihydrofurans, coumarines, quinolines, oxathiines, dibenzoxepines, benzocarbazoles and cyclobutane-fused pyrrolidines, inter alia (Gay et al. 2010; Mantovani et al. 2014; Wang et al. 2011; Sonehara et al. 2017; Yao et al. 2012, Sivaraman and Perumal 2014; Teske and Plietker 2016; Fürstner et al. 2008; Gudmundsson et al. 2018, Kramm et al. 2018; Boominathan et al. 2015; Sreedevi et al. 2010).
A number of polyheterocycles have been synthesized by a versatile cascade process between alkynoic acids and functionalized amines. It has been proposed that a metal-catalyzed cycloisomerization of the alkynoic acid followed by a nucleophilic attack by the amine on the resulting lactone generates a ketoamide intermediate that upon intramolecular condensation and subsequent reaction with the additional nucleophilic center provides complex polycyclic compounds structurally related to several alkaloids with COX-1 and COX-2 inhibiting and bronchodilatory activities such as Vasicine, Mackinazoline, Batracyclin and Tryptanthrine (Kshirsagar 2015; Nepali et al 2012). Gold catalysts have been predominantly used for this purpose (Yang et al. 2007; Cadierno 2020; Zhou et al. 2010a, b, 2011; Feng et al. 2010, 2012; Ji et al. 2013; Li et al. 2013; Qiao et al. 2019; Jia et al. 2019; Patil et al. 2010, 2012, 2013) though copper-catalyzed (Naidu and Reddy 2016) and ruthenium-catalyzed (Zheng et al. 2020) methodologies have been also reported. However, to the best of our knowledge, there is no example of the use of iron in such transformations that provide a direct access to potentially pharmacologically active compounds. Indeed, no cascade cycloisomerization/ketoamide formation/double intramolecular cyclocondensation has been described for iron catalysts so far. Following our research on iron-promoted hydroamidations and on other metal-catalyzed tandem processes involving alkynes (Herrero et al. 2012; Moure et al. 2014) we envisioned that iron salts could promote such a domino process, thus procuring a more sustainable alternative to the use of precious metals (Fig. 1). Herein we wish to report our results on the iron-catalyzed cascade reaction between alkynoic acids and functionalized amines.
Experimental
General. Commercially available reagents were used throughout without purification unless otherwise stated. 1H and 13C NMR spectra were recorded on a Bruker AC-300 instrument (300 MHz for 1H and 75.4 MHz for 13C) at 20 °C. Chemical shifts (δ) are given in ppm downfield from Me4Si and are referenced as internal standard to the residual solvent (unless indicated) CDCl3 (δ = 7.26 for 1H and δ = 77.00 for 13C). Coupling constants, J, are reported in hertz (Hz). Melting points were determined in a capillary tube and are uncorrected. TLC (thin-layer chromatography) was carried out on SiO2 (silica gel 60 F254, Merck), and the spots were located with UV light. Flash chromatography was carried out on SiO2 (silica gel 60, Merck, 230–400 mesh ASTM).
General Procedure A: A screw-capped tube was charged with amine (0.50 mmol), alkynoic acid (0.75 mmol), FeBr2 (0.05 mmol), pyridine (0.20 mmol) and toluene (1 mL). The reaction mixture was heated to 150 °C for 24 h and allowed to cool to room temperature. The resulting mixture was purified by silica gel flash column chromatography to afford the corresponding compound.
General Procedure B: A screw-capped tube was charged with amine (0.50 mmol), alkynoic acid (0.75 mmol), FeBr2 (0.05 mmol) and pyridine (0.20 mmol). The reaction mixture was heated to 100 °C for 24 h and allowed to cool to room temperature. The resulting mixture was purified by silica gel flash column chromatography to afford the corresponding compound (see Supplementary Material section for further details).
Results and discussion
Optimization of reaction conditions
2-Aminobenzylamine 1a and 4-pentynoic acid 2a were chosen as model substrates for the optimization of reaction conditions. After a number of initial experiments, we observed that indoloquinazolinone 3a was obtained with moderate to good yields after heating the substrates in the presence of iron(III) chloride hexahydrate and pyridine in different solvents (Table 1, entries 1–4). The best results were achieved using toluene and ethanol as reaction media (entries 1–2). We resolved to use toluene as solvent for the following assays in order to avoid the possible competition with ethanol if less nucleophilic amine derivatives were used. Other organic and inorganic bases (Na2CO3, Cs2CO3, DMAP) were also assayed but provided significantly inferior results.
The role of the catalyst seemed critical since the heterocycle was obtained in low yield in the absence of any metal source (entry 9). The formation of product 3a under the latter conditions could be explained by considering a thermally induced partial cycloisomerization of the acid. Regarding the catalyst system, FeBr2 provided the best results (entries 5–7) probably due to its superior carbophilia. In fact, aluminum trichloride, a more acidic Lewis acid, turned out to be not a suitable metal source (entry 8). Unfortunately, the decrease of the catalyst loading reduced considerably the reaction yield (entry 15 vs 11 and 16 vs 12).
Besides, a catalytic amount of pyridine had a slightly positive effect (entry 7 vs 5, 13 vs 11 and 14 vs 12), and a more important variable was the concentration of the reaction mixture (entries 10–12). Higher concentrations resulted in better results (entry 11) and interestingly, 3a was also isolated in good yields when reactants were heated in the absence of solvent at a much lower temperature (100 °C, entry 12). Therefore we chose FeBr2 (10 mol%) and pyridine (40 mol%) in toluene at 150 °C or, alternatively, at 100 °C with no solvent as the optimized conditions to explore the scope of the reaction.
Substrate
Different diamines 1 were initially reacted with 4-pentynoic acid 2a. To our delight, in all cases target pyrrolidinone-fused compounds 3a-n were selectively obtained in moderate to excellent yields (Fig. 2). The nucleophilicity of the functional groups of substrates 1 was the key to rationalizing the obtained structure. As expected, the more nucleophilic functional group would be responsible for the opening of the furanone generated by cycloisomerization of 4-pentynoic acid, being the N-acyliminium ion intermediate attacked by the second nucleophile. In the case of 2-amino substituted anilines, the difference in nucleophilicity between both amino groups plays a pivotal role in the outcome of the reaction. In fact, an excellent yield for the tricycle was obtained from N-fenil-1,2-diaminobenzene 1d, whereas 1,2-diaminobenzene 1b provided a complex mixture of products from which heterocycle 3b was isolated in moderate yield. In this case, the similar reaction rate of both amino groups might result in reaction with two furanone units leading to a diamide intermediate that could not evolve to the product. In this regard, it should be pointed out the completely regioselective access to pyrroloimidazopyridinone 3c from 2,3-diaminepyridine 1c, thus proving not only our hypothesis above but also that the presence of a pyridine ring in the dinucleophile did not hinder the progress of the reaction. Another interesting feature of the reaction from N-phenyl-1,2-diaminobenzene 1d is the fact that secondary amines can participate in the reaction as long as they are weaker nucleophiles than the primary amine moiety so that a secondary amide intermediate, unable to evolve to the final product, is not formed.
Other o-funcionalized aniline derivatives also reacted with 4-pentynoic acid to provide selectively polycycles 3e–3h with excellent yields. Except for thiadiazine 3e, better results were observed when the reaction was carried out in toluene at 150 °C. Following the same trend as with diamines, the aminolysis of the alkylidene lactone took place initially, being the carboxamide, carboxi, sulfonamide or mercapto group responsible for the second nucleophilic attack. Besides, reaction with N-substituted amides provided tetrahydropyrroloquinazoline-1,5-diones 3i-3n although in lower yields, especially in the case of bulky substituents on the amide nitrogen.
The optimized conditions were then applied to other alkynoic acids. Pyridoquinazolinone 3o was obtained from 2-aminobenzylamine and 5-hexynoic acid but in lower yield than pyrroloquinazolinone 3a, seemingly due to the more difficult cycloisomerization of 5-hexynoic acid (Bunce and Nammalwar 2011). In contrast, better results were obtained from 2-ethynilbenzoic acid, which was supposed to react via formation of 3-methyleneisobenzofuran-1(3H)-one, thus providing tetracycles 3p–3r. The methodology also allowed us to prepare selectively quinazolines 3s and 3t from non-terminal alkynes 2-phenylethynyl benzoic acid and 5-phenyl-4-pentynoic acid respectively. However, neat heating at 100 °C was required to achieve moderate yields of these two benzylated derivatives. In addition to the spectroscopic analysis data, X-ray crystallographic analysis of derivatives 3g and 3p confirmed the assigned structure and therefore underpinned the proposed regioselectivity.
As mentioned before, the related cascade reactions described in the literature have been carried out using Au(I) (often Au(I)-Ag(I)), Cu(II) and Ru(II) catalysts. In addition to the higher cost of these precious and semiprecious metal catalyst systems (4–25 times as much as FeBr2/py/PhMe), the extensive mining of natural resources for the extraction of the aforementioned scarce metals should not be ignored. Iron is the 5th most abundant element found on Earth crust, and in contrast to gold, copper or ruthenium, iron compounds and salts exhibit low toxicity or are practically harmless (Egorova and Ananikov 2017). Although pyridine is used in our protocol, only a catalytic amount (40 mol%) is required to get selectively target tri- and tetracyclic products 3a–t. Moreover, except for pyrido[2,1-b]quinazolin-9-one 3o, comparable and even superior yields were obtained for polyheterocycles 3a–b, 3f, 3h and 3o–q in comparison with previously reported procedures (see Table S1 for a comparative analysis).
Mechanistic studies
With the aim of gathering information about the mechanism of the reaction catalyzed by FeBr2, furanone 4 was reacted with o-aminobenzylamine (Fig. 3a). The reaction in the presence of the iron salt provided tricycle 3a in very good yield, hence supporting our hypothesis on the participation of an enol lactone intermediate as a result of an initial cycloisomerization step. The significantly lower yield obtained in the absence of FeBr2/pyridine pointed to the assistance of the metal catalyst in the subsequent steps. Conversely, a basic reaction environment (Cs2CO3) hinders the progress of the reaction, probably by preventing dehydration and formation of the key N-acyliminium intermediate III (Fig. 3d). We also confirmed that the combination of FeBr2 and pyridine was able to cause the cycloisomerization of 4-pentynoic acid, since isomeric furanone 5 was isolated when 4-pentynoic acid was heated with FeBr2/pyridine in the absence of the dinucleophile (Fig. 3b).
The formation of 5-phenylmethylaminopyrrolidinone 8 and the dimeric compound 9 from the reaction between 4-pentynoic acid and benzyl amine (Fig. 3c) is consistent with the intermediacy of an N-acyliminium intermediate (Fig. 3c). Indeed, the above compounds 8–9 can be easily rationalized as products from the addition of benzylamine and the enamide in equilibrium with the N-acyliminium ion, respectively, to the said iminium intermediate (Figure S1 and Figure S2). Finally, isolation of N-phenyl-4-propynamide 7 was expectable considering the high temperature and the presence of a Lewis acid in the reaction media. Nevertheless, it should be pointed out that no amide derivative was detected when ortho-functionalized anilines or benzylamines were employed in these reactions.
On the basis of the results from the performed assays we propose that, like in other metal-catalyzed domino processes from alkynoic acids, the reaction would be initiated by the coordination of iron catalyst to the triple bond, thus facilitating the cycloisomerization of the acid to furanone intermediate I, which would generate ketoamide II by aminolysis with 1a. The activation of the ketone function at II by the iron salt would trigger the formation of highly reactive N-acyliminium ion III, which would undergo an intramolecular addition of the amine moiety to give pyrroloquinazolinone 3a (Fig. 3d).
Conclusion
We have developed a new catalytic system based on iron(II) bromide for the high-yielding synthesis of nitrogen, oxygen and sulfur heterocycles from alkynoic acids and functionalized amines. Considering the comparable yields obtained, the natural abundance of iron sources and the environmental concern over the exploitation of natural resources, we conclude that such an inexpensive, accessible and low-toxicity metal salt as iron(II) bromide offers a viable and more sustainable alternative to the previously reported catalytic systems, based on precious or semiprecious metals. With regard to the reaction mechanism, a number of experiments involving reaction with plausible intermediates or in the absence of the functionalized amine shed light on the role of the catalyst system. Thus, the reported one-step methodology probably takes place through a cycloisomerization/nucleophilic addition/cyclodehydration cascade where the iron catalyst is involved not only in the initial cycloisomerization step but is essential for achieving good results in this straightforward access to pyrrolo and isoindolo-fused heterocycles.
Availability of data and materials
Supporting information for characterization of synthesized compounds is provided including NMR data.
References
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, New York
Bauer I, Knölker HH (2015) Iron catalysis in organic chemistry. Chem Rev 115:3170–3387
Bolm C, Legros J, Le Paih J, Zani L (2004) Iron-catalyzed reactions in organic synthesis. Chem Rev 104:6217–6254
Boominathan SSK, Senadi GCh, Vandavasi JK, Chen JY-F, Wang J-J (2015) An iron-catalyzed cascade approach to benzo[b]carbazole. Synthesis followed by 1,4-sulfonyl migration. Chem Eur J 21:3193–3197
Bunce RA, Nammalwar B (2011) New conditions for synthesis of (6)-2-monosubstituted and (6)-2,2-disubstituted 2,3-dihydro-4(1h)-quinazolinones from 2-nitro- and 2-aminobenzamide. J Heterocycl Chem 48:991–997
Cadierno V (2020) Gold-catalyzed addition of carboxylic acids to alkynes and allenes: valuable tools for organic synthesis. Catalysis 10:1206–11242
Casnati A, Lanzi M, Cera G (2020) Recent advances in asymmetric iron catalysis. Molecules 25:3889–3919
Crichton R (2016) Iron metabolism: from molecular mechanisms to clinical consequences. Wiley, Chichester
DaBell P, Thomas SP (2020) Iron catalysis in target synthesis. Synthesis 52:949–963
Danz H, Stoyanova S, Thomet OAR, Simon HU, Dannhardt G, Ulbrich H, Hamburger M (2002) Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Med 68:875–880
Egorova KS, Ananikov VP (2017) Toxicity of metal compounds: knowledge and myths. Organometallics 36:4071–4090
Expert D (2012) Iron, an element essential to life. In: Expert D, O’Brian MR (eds) Molecular aspects of iron metabolism in pathogenic and symbiotic plant-microbe associations. Springer, Dordrecht, pp 1–86
Feng E, Zhou Y, Zhang D, Zhang L, Sun H, Jiang H, Liu H (2010) Gold(I)-catalyzed tandem transformation: a simple approach for the synthesis of pyrrolo/pyrido[2,1-α][1,3]benzoxazinones and pyrrolo/pyrido[2,1-a]quinazolinones. J Org Chem 75:3274–3282
Feng E, Zhou Y, Zhao F, Chen X, Zhang L, Jiang H, Liu H (2012) Gold-catalyzed tandem reaction in water: an efficient and convenient synthesis of fused polycyclic indoles. Green Chem 14:1888–1895
Fürstner A, Majima K, Martin R, Krause H, Kattnig E, Goddard R, Lehmann ChW (2008) A cheap metal for a “noble” task: preparative and mechanistic aspects of cycloiomerization and cycloaddition reactions catalyzed by low-valent iron complexes. J Am Chem Soc 130:1992–2004
Gay RM, Manarin F, Scheneider CC, Barancelli DA, Costa MD, Zeni G (2010) FeCl3-diorganyl dichalcogenides promotes cyclization of 2-alkynylanisoles to 3-chalcogen benzo[b]furans. J Org Chem 75:5701–5706
Gudmundsson A, Gustafson KPJ, Mai BKM, Himo F, Bäckvall JE (2018) Efficient formation of 2,3-dihydrofurans via iron-catalyzed cycloisomerization of α-allenols. ACS Catal 8:12–16
Guđmundsson A, Bäckvall J-E (2020) On the use of iron in organic chemistry. Molecules 25:1349–1378
Herrero MT, Diaz de Sarralde J, SanMartin R, Bravo L, Dominguez E (2012) Cesium carbonate-promoted hydroamidation of alkynes: Enamides, indoles and the effect of iron(III) chloride. Adv Synth Catal 354:3054–3064
Ji X, Zhou Y, Wang J, Zhao L, Jiang H, Liu H (2013) Au(I)/Ag(I)-catalyzed cascade approach for the synthesis of benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinones. J Org Chem 78:4312–4318
Jia X, Li P, Liu X, Lin J, Chu Y, Yu J, Wang J, Liu H, Zhao F (2019) Green and facile assembly of diverse fused N-heterocycles using gold-catalyzed cascade reactions in water. Molecules 24:988–1018
Kramm F, Teske J, Ullwer F, Frey W, Plietker B (2018) Annelated cyclobutannes by fe-catalyzed cycloisomerization of enyne acetates. Angew Chem Int Ed 57:13335–13338
Kshirsagar UA (2015) Recent developments in the chemistry of quinazolinone alkaloids. Org Biomol Chem 13:9336–9352
Li Z, Li J, Yang N, Chen Y, Zhou Y, Ji X, Zhang L, Wang J, Xie X, Liu H (2013) Gold(I)-catalyzed cascade approach for the synthesis of tryptamine-based polycyclic scaffolds as 1-adrenergic receptor antagonists. J Org Chem 78:10802–10811
Ludwig JR, Schindler CS (2017) Catalyst: sustainable catalysis. Chem 2:313–316
Mantovani AC, Goulart TAC, Back DF, Menezes PH, Zeni G (2014) Iron (III) chloride and diorganyl diselenides-mediated 6-endo-dig cycliza-tion of arylpropiolates and arylpropiolamides leading to 3-organoselenyl-2h-coumarins and 3-organoselenyl-quinolinones. J Org Chem 79:10526–10536
Moure MJ, SanMartin R, Domínguez E (2014) Copper pincer complexes as advantageous catalysts for the heteroannulation of o-halophenols and alkynes. Adv Synth Catal 356:2070–2080
Naidu S, Reddy SR (2016) Copper-catalyzed tandem reactions in ionic liquid: an efficient reusable catalyst and solvent media for the synthesis of fused polyheterocyclic compounds. RCS 6:62742–62746
Nepali K, Sharma S, Ojha R, Dhar KL (2012) Vasicine and structurally related quinazolines. Med Chem Res 22:1–15
Patil NT, Mutyala AK, Lakshmi PGVV, Gajula B, Sridhar B, Pottireddygari GR, Rao TP (2010) Au(I)-catalyzed cascade reaction involving formal doble hydroamination of alkynes bearing tethered carboxylic groups: an easy access to fused dihydrobenzimidazoles and tetrahydroquinazolines. J Org Chem 75:5963–5975
Patil NT, Lakshmi PGVV, Sridhar B, Patra S, Bhadra MP, Patra CR (2012) New linearly and angularly fused quinazolinones: synthesis through Gold(I)-catalyzed cascade reactions and anticancer activities. Eur J Org Chem 2012:1790–1799
Patil NT, Shinde VS, Sridhar B (2013) Relay catalytic branching cascade: a technique to access diverse molecular scaffolds. Angew Chem Int Ed 52:2251–2255
Piontek A, Bisz E, Szostak M (2018) Iron-catalyzed cross-coupling in the synthesis of pharmaceuticals: in pursuit of sustainability. Angew Chem Int Ed 57:11116–11128
Qiao J, Jia X, Li P, Liu X, Zhao J, Zhou Y, Wang J, Liu H, Zhao F (2019) Gold-catalyzed rapid construction of nitrogen-containing heterocyclic compound library with scaffold diversity and molecular complexity. Adv Synth Catal 361:1419–1440
Rana S, Biswas JP, Paul S, Paik A, Maiti D (2021) Organic synthesis with the most transition metal-iron: from rust to multitasking catalysts. Chem Soc Rev 50:243–472
Sivaraman M, Perumal PT (2014) Synthesis of 4-methyl-2,3-disubstituted quinolone scaffolds via environmentally bening Fe (III) catalyzed sequential condensation, cyclization and aromatization of 1,3-diketone and 2-ethynylaniline. RCS Adv 4:52060–52066
Sonehara T, Murakami S, Yamazaki S, Kawatasura M (2017) Iron-catalyzed intermolecular hydrothiolation of internal alkynes with thiosalicyclic acids, and sequential intramolecular cyclization reaction. Org Lett 19:4299–4302
Sreedevi R, Saranya S, Rohit KR, Anilkumar G (2010) Recent trends in iron-catalyzed reactions towards the synthesis of nitrogen-containing heterocycles. Adv Synth Catal 361:2236–2249
Teske J, Plietker B (2016) A redox-neutral Fe-catalyzed cyclioisomerization of enyne acetates. ACS Catal 6:7148–7151
Wang Z-Q, Lei Y, Zhou M-B, Chen G-X, Li J-H (2011) Iron-mediated [3+2] or [3+3] annula-tion of 2-(2-(ethynyl)phenoxy)-1-arenylethanones: selective synthesis of indeno[1,2-c]chromenes and 5h-naphtho[1,2-c]chromenes. Org Lett 13:14–17
Wei D, Darcel Ch (2019) Iron catalysis in reduction and hydrometalation reactions. Chem Rev 119:2550–2610
Yang T, Campbell L, Dixon DJ (2007) A Au(I)-catalyzed N-acyl iminium ion cyclization cascade. J Am Chem Soc 129:12070–12071
Yao Ch, Qin B, Zang H, Lu J, Wang D, Tu Sh (2012) One-pot solvent-free synthesis of quinolones by C–H activation/C–C bond formation catalyzed by recyclable iron(III) triflate. RCS Adv 2:3759–3764
Zhang J, Wang S, Feng Z (2020) Iron-catalyzed cross-coupling reactions for the construction of carbon-heteroatom bonds. Asian J Org Chem 9:1519–1531
Zheng Y, Liu J, Lei X (2020) Ru-Catalyzed cascade reactions of α,ω-alkynoic acids and arylethylamines towards the synthesis of aryl-fused heterocycles. Org Chem Front 7:660–665
Zhou Y, Ji X, Liu G, Zhang D, Zhao L, Jiang H, Liu H (2010a) Gold(I)-catalyzed cascade for synthesis of pyrrolo[1,2-a:2´,1´-c]-/pyrido[2,1-c]pyrrolo[1,2-a]quinoxalinones. Adv Synth Catal 352:1711–1717
Zhou Y, Zhai Y, Ji X, Liu G, Feng E, Ye D, Zhao L, Jiang H, Liu H (2010b) Gold(I)-catalyzed one-pot tandem coupling/cyclization: An efficient synthesis of pyrrolo-/pyrido[2,1-b]benzo[d][1,3]oxazin-1-ones. Adv Synth Catal 352:373–378
Zhou Y, Li J, Ji X, Zhou W, Zhang X, Qian W, Jiang H, Liu H (2011) Silver- and gold-mediated domino transformation: a strategy for synthesizing benzo[e]indolo[1,2-a]pyrrolo/pyrido[2,1-c][1,4]-diazepine-3,9-diones. J Org Chem 76:1239–1249
Acknowledgements
Technical and human support provided by SGIker of UPV/EHU is gratefully acknowledged.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Basque Government (IT1405-19) and the Spanish Ministry of Economy and Competitiveness (CTQ2017-86630-P). JD thanks the Basque Government for a predoctoral scholarship. Finally, technical and human support provided by SGIker of UPV/EHU is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
MTH, RS and GU contributed to searching and collating of the relevant literature and the proofreading of the document. Investigation, experimental and analysis were carried out by JD, EA, AS and YR. MTH and RS conceptualized and supervised the study and wrote the body of the article. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Authors declare no competing financial interest.
Consent for publication
All the authors have seen and approved the final version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Sarralde, J.D., Astobieta, E., Sevilla, A. et al. Iron-catalyzed cascade synthesis of nitrogen polycycles from alkynoic acids and functionalized amines. Environ Chem Lett 20, 3421–3427 (2022). https://doi.org/10.1007/s10311-022-01477-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01477-y