Abstract
Background
The acronym FIRES stands for febrile infection-related epileptic syndrome, which is a rare epileptic syndrome in the pediatric population. The initial presentation of FIRES is similar to febrile seizures (FS). Both start after a febrile episode; however, in FIRES the epileptic seizure evolves into a super refractory status epilepticus within days despite appropriate treatment. FIRES needs to be diagnosed early and treated by a multidisciplinary team to control the status epilepticus (SE) as fast as possible. Limiting the duration of the SE is paramount for the prevention of catastrophic sequelae such as severe neurologic disabilities or even death.
Objective/Conclusion
We describe possible pathophysiological mechanisms and summarize important clinical features of FIRES. The aim of this review is to raise awareness, foster early recognition and improve neurologic long-term outcomes. Moreover, we propose a diagnostic approach and list therapeutic options providing an algorithm.
Zusammenfassung
Hintergrund
Das Akronym FIRES steht für „febrile infection-related epileptic syndrome“ und gehört zu den seltenen Epilepsiesyndromen im Kindesalter. Wie bei Fieberkrämpfen (FK) kommt es auch bei FIRES initial zu einem fieberassoziierten Anfall, der innerhalb von wenigen Tagen zu einem supertherapierefraktären Status epilepticus (SE) führt – trotz adäquater Therapie. FIRES sollte rasch diagnostiziert werden und von einem multidisziplinären Team betreut werden, um den SE frühzeitig unter Kontrolle zu bringen und somit mögliche schwerwiegende neurologische Folgen oder Tod zu vermeiden.
Ziel und Schlussfolgerung
Für die Sensibilisierung und somit Früherkennung dieser Krankheit wurden in der vorliegenden Arbeit mögliche pathophysiologische Mechanismen und die wichtigen klinischen Symptome zusammengefasst. Ein diagnostischer Ansatz und mögliche therapeutische Optionen wurden in einer algorithmisch illustrierten Grafik dargestellt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Febrile infection-related epileptic syndrome (FIRES) is a rare epileptic syndrome in the pediatric population with an estimated incidence of 1 in 1 million. The initial presentation of FIRES is similar to febrile seizures (FS). In contrast to mostly benign and self-limiting FS, in patients with FIRES, seizures evolve into a refractory status epilepticus. The course of the disease is therefore complicated and the outcome much worse. Here, we highlight possible pathophysiological mechanisms and the clinical symptoms, propose a diagnostic approach, and review the therapeutic options. Our aim is to raise awareness and foster early recognition of FIRES in order to improve neurologic outcome.
Introduction
More than 60 years ago, 16 cases [31] of acute encephalopathy of obscure origin in infants and children after a febrile infection were described for the first time. During the following years similar cases using different names were published: devastating epileptic encephalopathy in school-aged children (DESC); acute encephalitis with refractory, repetitive partial seizures (AERRPS); or catastrophic epileptic encephalopathy presenting with acute onset of intractable seizures [23]. The lack of standard terminology made early recognition of the disease difficult, which resulted in delayed diagnosis.
Definition
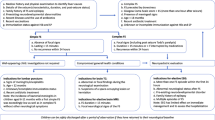
In 2010 the ACRONYM FIRES was coined by a German group [41] and 8 years later an international group of experts proposed a consensus definition [14]. FIRES was defined as a subcategory of new-onset refractory status epilepticus (NORSE). In contrast to NORSE, patients suffering from FIRES always present with a preceding febrile infection starting between 2 weeks and 24 h prior to onset of SE (see Fig. 1 Algorithm Graph: History), with or without fever at onset of SE [14]. Hence, fever remains a sine qua non for the diagnosis. Nevertheless, most patients with NORSE also present with fever [10, 14] and in 60% of NORSE patients a mild preceding infection is present. In the past the term NORSE was reserved for adults and FIRES for children with fever. Today, the diagnosis FIRES is used across all ages.
Incidence
The annual incidence of FIRES in a German population was estimated as 1 case per 1,000,000 [42]. In a multicenter American study, 16 out of 92 patients with refractory SE and no previous history of epilepsy met the criteria of FIRES [37]. In smaller countries like Switzerland and Austria, the number of FIRES patients is limited to 5–8 cases per year. For better guidance, an algorithm may support the treating team in not only making the diagnosis but also choosing the best treatment option. So far more than 220 cases have been published (Table 1: FIRES cases from 1961 to 2022).
Clinical features
The syndrome starts with a febrile illness between 2 weeks and 24 h prior to onset of SE in previously healthy children (see Algorithm Graph: History). Unlike FIRES, children with FS [37] are younger and the time onset between fever and epileptic seizure is usually less than 24 h. Moreover, body temperature in FIRES is lower, the SE lasts longer (more than 48 h) and is usually refractory to multiple antiseizure drugs (ASD). FIRES mostly occurs in children at a median age of 6–8 years [23, 32, 43]. To date, no affected siblings have been reported. Rarely family members have epilepsy [20, 41]. FIRES is characterized by three phases. A prodromal phase lasting for 13 days with febrile illness; mostly of the respiratory tract followed by gastrointestinal infection [20, 23]. Beyond that seizures develop and get more frequent, evolving into refractory SE (see Fig. 1 Algorithm Graph: Clinical Presentation). At the time of SE, most FIRES children have recovered from their initial illness. Fever might still be present but often a fever-free interval occurs. The third phase is a lifelong chronic stage with patients being on multiple ASD and having different grades of disability. Seizures are usually multifocal or generalized [43] with full or impaired awareness evolving into super refractory SE counting up to several hundred seizures a day. Clinically, there is a frontotemporal predominance in both cerebral hemispheres [23, 41]. Common EEG features as possible biomarkers for early recognition have been published [8]:
-
1.
Initial seizures are brief, infrequent and gradually evolve to SE.
-
2.
Beta delta complexes resemble extreme delta brushes.
-
3.
Seizures generally begin with prolonged focal (Fig. 2) fast activity, followed by gradual appearance of well-formed rhythmic spikes or spike wave complexes (Fig. 3a–c).
a aEEG: Delta brushes in FIRES: paroxysmal beta–delta complex consisting of 15–18-Hz beta activity superimposed over 1–3 Hz delta (black arrows). b aEEG: Beginning of a right hemispheric seizure (red arrow) and later a left hemispheric seizure (blue arrow). c aEEG: Continuation of both right (red arrow) and left (blue arrow) independent focal seizures
Diagnosis
FIRES remains a clinical diagnosis because no biological markers or genetic testing exists. Ideally, it should be recognized within the first 48–72 h. FIRES is a diagnosis of exclusion hence extensive work-up is needed (see Fig. 1 Algorithm Graph: Diagnosis) to rule out other differential diagnoses such as epilepsy, encephalitis, and encephalopathy [43]. The prerequisites for the diagnosis include no central nervous system infection or autoimmune encephalitis, no structural cause, and the absence of a genetic condition [21]. To encourage early recognition, several diagnostic flowcharts have been proposed ([20, 21, 34]; see Fig. 1 Algorithm graph: Diagnosis). The CSF is usually unremarkable with few exemptions showing mild pleocytosis [20, 43] but no pathogen [14, 20, 42]. Initial brain magnetic resonance imaging (MRI) is normal in more than half of the cases [5, 20, 23]. During the acute stage, 25% [5] have signal abnormalities in the temporal lobe and rarely other abnormalities in the basal ganglia and in the thalami. Over time there is a progression to diffuse atrophy of the cerebrum and cerebellum with sclerosis of the hippocampi [5, 20, 43] and subsequent ventriculomegaly. Brain biopsy is rarely performed showing mostly gliosis and seldom leptomeningeal inflammatory infiltrates [23, 41]. Whole-exome and HLA sequencing in FIRES did not identify any pathogenic variant in established genes for epilepsies or any prominent HLA alleles [13].
Etiology
The etiology of FIRES remains unknown. Over the past few decades, the following hypotheses have been discussed: (a) infections, (b) autoimmune-mediated, (c) inflammation-mediated, (d) genetic, or (e) metabolic [14, 20, 23, 43].
A few cases tested positive for mycoplasma or adenovirus, although these pathogens were not primary responsible for the current disease; however, in most of the cases no pathogens are found. Furthermore, CSF protein might also be increased as a breakdown of the blood–brain barrier (BBB). Infection as the underlying reason for FIRES therefore seems unlikely.
Elevated autoimmune antibodies have been reported in FIRES cases [18, 20, 23] suggesting an autoimmune-mediated origin [43]. However, these patients might instead fit the diagnosis of NORSE [10]. One of the largest systematic reviews [20] found 11.4% of the patients to be positive for either anti-glutamate receptor antibodies or anti-GAD antibody and IL6/IL8 in CSF. Single case reports detected PCDH19, anti-SMA, and SCN2A [20]. The brief time gap between febrile illness and SE [23] and the disappointing response to immunotherapy [20, 41, 43] does not support the aforementioned theory.
The time delay [14] is more suggestive of a post-infectious triggering process than an infectious disease itself [23]. An inflammation-mediated mechanism as the leading process was also hypothesized [20, 23, 41]; however, a lack of response to immune modulators [43] and the absence of inflammation on CSF, MRI, and brain biopsy speaks against it.
Proinflammatory molecules (cytokines, chemokines) during febrile illness might lower the seizure threshold by modifying ion channel functions and promoting neuronal excitability with a certain delay [23, 43]. These molecules prime the activation of innate immunity mechanisms in glia cells, neurons, and cellular components of the BBB in seizure-prone areas, giving rise to a neuroinflammatory cascade. This process reflects the aforementioned time delay. In short, FIRES represents an inflammatory but not autoimmune-mediated encephalopathy with an immune response leading to intractable seizures [43]. An imbalance between pro- and anti-inflammatory molecules with increased intrathecal cytokines and chemokines has been postulated for the time delay [23, 43].
A double hit process between (a) an immune response to a febrile illness affecting the brain and (b) an intrinsic predisposition toward an auto-sustaining epileptogenic process was later proposed [38]. The mechanistic model of epileptogenesis for FIRES includes a systemic infection that activates the immune system. This again releases pro-inflammatory molecules and lowers seizure threshold. Then, SE not only releases pro-inflammatory cells, turning into a vicious circle, but also leads to chronic tissue damage ending in chronic epilepsy. Seizures trigger neuroinflammation and contribute to more seizure activities. This acts concomitantly to an acquired or genetic predisposed factor.
FIRES shows a proinflammatory alteration in CSF cytokine profiles with elevated interleukin IL-6, C‑X‑X motif chemokine ligand 8 (CXCL8), and C‑C motif ligand L3 and 4 (CCL4 and CCL3; [11, 20, 22]). The IL‑6 was more elevated in CSF than in serum [11], suggesting a CNS-specific inflammation. It remains unclear whether this alteration is primary or secondary to SE. The proinflammatory cytokine IL-1β is upregulated in brain tissue from drug-resistant epilepsy but not consistently elevated in CSF or serum [20]. The clinical utility of these cytokines in predicting response to immune therapy needs to be studied further.
Inconsistently elevated IL‑1 receptor antagonist (IL-1RA) levels in serum and CSF of FIRES means that IL-1RA is functionally compromised [4]. The IL-1RA gene mutations may play a role as a predisposing factor. In contrast to FIRES, a reduced level of IL-1RA was found in febrile SE, which might explain a genetic involvement in the production and function of this specific cytokine of FIRES.
An autoinflammatory thesis by an excessive activation of the microglial NLRP3 inflammasome /IL1 axis creating a proinflammatory and proconvulsive milieu has been discussed [29]. This might provoke a vicious cycle between inflammation and seizures. Children with FIRES also have impaired TLR3, TLR4, TLR7/8, and TLR9 responses that are related to weakened phagocytosis and lower T‑regulatory cells [16]. These patients may benefit from immunomodulatory therapy (steroids, intravenous immunoglobulins [IVIG]).
Differential diagnosis
Several differential diagnoses should be taken into consideration: FS, infectious encephalitis, posterior reversible encephalopathic syndrome (PRES), Dravet syndrome, familial acute necrotizing encephalopathy (ANE1) associated with a ran-binding protein2 (RNP2) mutation [27], Hashimoto’s disease, Alpers disease, and metabolic diseases [43]. To rule out some of these diagnoses, diagnostic tests are performed such as lumbar puncture, metabolic screening, genetic testing, thyroid hormone, and neuroimaging (see Fig. 1 Algorithm Graph: Diagnosis). The biphasic course and the refractory or rather challenging SE of FIRES patients makes the diagnosis of an encephalitis improbable. PCDH19 epilepsy can mimic FIRES [20] but can be distinguished by age, gender (usually younger and females), and by a good response to intravenous benzodiazepine. In both, a preceding viral illness can occur but PCDH19-affected children are characterized by a predominance of autism [23]. DRAVET syndrome shares similarities with FIRES but occurs much earlier in life, usually before the age of 1 year. In contrast to FIRES patients [35] who do not have a SCNA1 mutation, 75–80% of DRAVET patients are positive for the mutation [12, 35]. They often present with cluster of seizures rather than SE [14] with a normal EEG in the first 2 years. An intellectual disability was also reported after 1–3 years. In cases of POLG1 mutation, seizures are occipital and have a unilateral predominance in contrast to FIRES where seizure activity is frontotemporal and in both cerebral hemispheres [23, 42]. In summary, SCNA1-, PDCH19-, and POLG1-associated epilepsies have an earlier age of onset and SE is less severe. FIRES remains a diagnosis of exclusion. To rule out the aforementioned diseases, an extensive work-up including blood and CSF samples for infectious, autoimmune, metabolic, and genetic testing is required (see Fig. 1 Algorithm Graph: Diagnosis). Genetic testing normally takes some time, and therefore at the beginning children are treated independently of these results. In previous published case reports, however, genetic testing is often missing [20]. As there is still no specific biomarker for its diagnosis, the population might still be heterogenous.
Therapeutic options
Primary treatment goal and referral to specialized center
Today there is still no specific treatment available since evidence is lacking in guiding the choice, dosing, and timing [11, 33]. For each individual patient the best therapeutic option must be discovered by perhaps even trying several drugs without getting any effect. Primary goal of treatment [20, 33, 34, 37] is to control the SE and to avoid iatrogenic complications. Affected children should be referred to a specialized center with a multidisciplinary team of neuropediatricians, intensivists, neuroradiologists, and pharmacologists. Hospitalization time can vary from 14 to 208 days [25]. Most children need mechanical ventilation (median duration: 41 days; [23, 25]) and are admitted to the intensive care unit with a median stay from 20 to 40 days [37].
Acute phase: suppressing seizures
The acute phase is the most frustrating and vulnerable one for family members and the medical team. It also impacts the long-term outcome.
The fundamental approach is to suppress clinical and subclinical epileptic seizures by the adjustment of first- and second-line ASD (see Fig. 1 Algorithm Graph: Treatment) based on continuous EEG. Up to four to six ASD per day are often insufficient to stop seizures. Common reported adverse events of ASD are hepatic dysfunction and DRESS [20]. One third of children [10] end up requiring multiple anesthetics to achieve seizure control as conventional ASD are not enough [11, 23]. A high dose of barbiturate to induce burst coma suppression (BCS) seems to be the only effective therapeutic option [20, 23]. Multiple cycles up to 7 days are usually required [11, 20] and after tapering, seizures often reoccur. Commonly used anesthetics are midazolam infusion, propofol, thiopentone, pentobarbital, and ketamine. Prolonged burst suppression is associated with longer ICU stay, intubation, higher rate of complications, and worst cognitive outcome [23, 39]. Potential serious side effects of barbiturate such as hypotension, acidosis, infection, ileus, and change in potassium concentration must be monitored. Due to awareness of propofol infusion syndrome, this alternative option has been pushed to the back [2].
First-line and second-line treatments
Anti-inflammatory drugs are subdivided into first line (steroid, IVIG and PLEX) and second line (anakinra, cyclophosphamide, rituximab; [11, 20, 33]) showing mixed results [20, 43].
First-line treatment (see Fig. 1 algorithm graph: treatment)
Dosage recommendations are: steroid 10–30 mg/kg/day for 3–5 days, IVIG 0.4–2 g/kg over 3–5 days, and plasma exchange (PLEX) 2–4 exchanges on every other day [11, 20, 38]. Ritter et al. [34] suggest starting with i.v. methylprednisolone, then PLEX, followed by IVIG if there is no improvement. A treatment proposal from the latest workshop [21] agreed to start with methylprednisolone and/or IVIG. Starting simultaneously [20, 34, 43] with pulse steroids and ketogenic diet (KD) statistically improves outcome compared to PLEX and IVIG. The patient is contemporaneously on multiple ASD. Even if immunosuppressive treatments are applied early among FIRES, they show disappointing effects [14].
If no improvement after first-line treatment
If no improvement is noted, second-line treatment such as with anakinra can be administered for a minimum of 3–4 weeks. Whereas immune modulators such as tocilizumab or canakinumab may be considered after persisting seizures despite therapy with anakinra. Drugs were allocated to categories of effective (KD, cannabidiol, phenobarbital, clobazam) versus partial or ineffective (perampanel, vagal nerve stimulation [VNS], lacosamide) after SE [20]. As burst suppression is associated with worse outcome, GABAergic therapy, immune therapy, mild hypothermia, and KD are recommended during the acute phase.
Ketogenic diet
Ketogenic diet was shown to have a dramatic efficacy in FIRES since it is anti-inflammatory and anticonvulsant for acute and long-term epilepsy [1, 20]. In 2010, a positive response of a 4:1 KD was first achieved after an onset of 4–6 days and confirmed later by weaning off anesthetics within 15 days [14, 20]. This effect [20, 43] could not be replicated in the subsequent study. It remains unclear why KD is efficacious in FIRES. Nevertheless, KD should be considered as first-line treatment and started early [20, 21, 43] with close monitoring of lipids and liver function [8]. The concomitant use of propofol and KD [2] remains a contraindication.
Therapeutic hypothermia
Since fever exacerbates seizure activity, controlling body temperature with therapeutic hypothermia (TH) may be beneficial to suppress convulsion [23]. By protecting the BBB integrity and reducing pro-inflammatory cytokines, TH may work toward the control of SE [11]. In a retrospective Taiwanese [17] study, seven patients with FIRES were managed for a few days with hypothermia (34–35°) and showed a significantly shorter duration of seizures, a better long-term outcome, and lower incidence of later refractory chronic epilepsy. Complications of hypothermia were bradycardia and electrolyte imbalance. Only two FIRES patients treated [20] with moderate hypothermia showed mild cognitive impairment.
Other adjunctive non-medical options
Other adjunctive non-medical options include VNS and electroconvulsive therapy (ECT). In DRAVET VNS revealed a seizure reduction of more than 50% in nearly half of the patients [6]. Similar results were confirmed in a pediatric retrospective study [7] of more than 140 children. Observed VNS complications entail hoarseness, deep infection requiring device removal, pneumothorax, and superficial infections. In FIRES, to date one patient has reached seizure control with VNS after 42 days [30]. A toddler underwent ECT and needed 14 sessions of progressive doses ranging from 40 to 200 J to become seizure free. At 1 year follow-up, she had severe retardation and diffuse atrophy. [20]. Six FIRES patients were treated with add-on intrathecal steroids after measuring cytokines before and after treatment [15]. Before treatment, several levels of cytokines and chemokines were elevated, although only CXLC10 and neopterin decreased after dexamethasone. After a median of 5.5 days, anesthetics could be weaned off, hence leading to a reduction in the ICU stay and the duration of intubation.
Second-line treatment (see Fig. 1 algorithm graph: treatment)
In an international cohort, 25 FIRES patients [24] were treated with anakinra to achieve control of seizure after other treatments had failed. Anakinra was started at a median of 20 days after seizure onset with a dose of 3–5 mg/kg/day for 1 day up to 420 days. In almost three quarters of patients, a seizure reduction of over 50% was observed. As a recombinant analogue of the human endogenous antagonist of IL‑1 receptor type 1, it inhibits IL-1B (proinflammatory cytokine), which is involved in autoinflammatory disease [43]. One case report showed normalization in IL‑8 and IL‑6 levels along with a reduction in seizure frequencies after anakinra [20]. Because its association with a shorter duration of mechanical ventilation, length of ICU/hospital stay, and seizure reduction, it should be implemented early if first-line medication fails [21, 24, 44]. Known adverse events are infection, DRESS, and cytopenia (neutropenia). The beneficial response of anakinra supports a hyperactive IL-1-beta activity and/or functional IL-1ra deficiency as potential causes of FIRES. Anakinra has also been effective during the chronic phase [24] Nevertheless, seizures reoccurred after stopping the drug. Another second-line drug option is tocilizumab, a humanized monoclonal antibody IL‑6 receptor [19] showing anti- and pro-seizures effects [9]. In FIRES [3], two children treated with tocilizumab had a positive response. Also in NORSE, six of seven patients reached resolution of SE within 2–10 days after 2 weeks of treatment [19] and IL‑6 levels in CSF and serum normalized. Reported side effects included leukopenia and severe infections [19, 26]. The IL‑6 concentration may be increased in seizures but its role in ictogenesis remains unclear. Tocilizumab does not cross the BBB, but prolonged seizures may disrupt this and increase its permeability. It remains unclear whether inflammation is primary or secondary to seizures. In FIRES, inflammatory cytokines and interleukins are superior compared to in afebrile SE and refractory epilepsies, supporting the presence of neuroinflammation [20, 22]. Evidence taken from the febrile SE literature suggests a possible correlation with outcomes [40].
Alternative treatment options (see Fig. 1 algorithm graph: treatment)
Continuous magnesium-sulfate infusion [43] up to 30 mg/kg/h used as rescue treatment in two FIRES cases showed decreased seizure frequency in one of them. Other single cases have been published on drugs such as perampanel, lidocaine infusion, and ketamine infusion [20]. It remains elusive if the effect of perampanel is coincidental or normal to the disease course [28].
Treatment during chronic phase
The chronic phase of FIRES resembles the “difficult to treat” epilepsy, and children rarely become seizure free. Cannabidiol and anakinra are recommended for this phase [24, 43]. By decreasing glutamate and gamma-aminobutyric acid synaptic transmission in the brain, cannabidiol also showed an improvement in seizure frequency and duration during the acute phase [11]. Other options involve rituximab and epilepsy surgery. Each drug or therapeutic modality is added on an already established therapy [26]. The concomitant use of different treatment modalities and their questionable effectiveness make a clear therapeutic recommendation difficult.
Despite the disappointing effect of first-line immunotherapies, they should be considered during the first week [36, 43] followed by second-line drugs if the former fail.
Practical conclusion
-
Febrile infection-related epileptic syndrome (FIRES) is associated with high mortality and morbidity. The mortality rate during acute and chronic phase ranges from 10 to 12%. Causes of death are multiorgan failure, hypotension, intracranial hemorrhage, acute hepatitis, respiratory failure, sepsis, and cardiac arrest
-
Individual outcome is difficult to predict but, in most cases, chronic epilepsy without a silent period occurs. More than half of FIRES children are on multiple antiseizure drugs, but only few become seizure free
-
Cognitive impairment can range from mild to severe with neuropsychological deficits such as attention, speech, and executive functioning issues.
-
Young age and longer burst coma suppression were associated with poor cognitive outcome and a more severe course of disease. By contrast, a good outcome was seen in children treated early with ketogenic diet.
-
In a long-term follow-up study, all seven patients with FIRES had refractory seizures, while four of them had moderate-to-severe intellectual impairment, two were borderline, and one died
References
Appavu B, Vanatta L, Condie J et al (2016) Ketogenic diet treatment for pediatric super-refractory status epilepticus. Seizure 41:62–65
Baumeister F, Oberhoffer R, Liebhaber G et al (2004) Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics 35:250–252
Cantarín-Extremera V, Jiménez-Legido M, Duat-Rodríguez A et al (2020) Tocilizumab in pediatric refractory status epilepticus and acute epilepsy: Experience in two patients. J Neuroimmunol 340:577142
Clarkson BD, Lafrance-Corey RG, Kahoud RJ et al (2019) Functional deficiency in endogenous interleukin‑1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Ann Neurol 85:526–537
Culleton S, Talenti G, Kaliakatsos M et al (2019) The spectrum of neuroimaging findings in febrile infection-related epilepsy syndrome (FIRES): a literature review. Epilepsia 60:585–592
Dibue-Adjei M, Fischer I, Steiger H‑J et al (2017) Efficacy of adjunctive vagus nerve stimulation in patients with Dravet syndrome: a meta-analysis of 68 patients. Seizure 50:147–152
Elliott RE, Rodgers SD, Bassani L et al (2011) Vagus nerve stimulation for children with treatment-resistant epilepsy: a consecutive series of 141 cases. J Neurosurg Pediatr 7:491–500
Farias-Moeller R, Bartolini L, Staso K et al (2017) Early ictal and interictal patterns in FIRES: the sparks before the blaze. Epilepsia 58:1340–1348
Gaspard N (2019) A new hose to Extinguish the fires? Epilepsy Curr 19:86–87
Gaspard N, Foreman BP, Alvarez V et al (2015) New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology 85:1604–1613
Gaspard N, Hirsch LJ, Sculier C et al (2018) New-onset refractory status epilepticus (NORSE) and febrile infection–related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia 59:745–752
Harkin LA, Mcmahon JM, Iona X et al (2007) The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain 130:843–852
Helbig I, Barcia G, Pendziwiat M et al (2020) Whole-exome and HLA sequencing in Febrile infection-related epilepsy syndrome. Ann Clin Transl Neurol 7:1429–1435
Hirsch LJ, Gaspard N, Van Baalen A et al (2018) Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia 59:739–744
Horino A, Kuki I, Inoue T et al (2021) Intrathecal dexamethasone therapy for febrile infection-related epilepsy syndrome. Ann Clin Transl Neurol 8:645–655
Hsieh M‑Y, Lin J‑J, Hsia S‑H et al (2020) Diminished Toll-like receptor response in febrile infection-related epilepsy syndrome (FIRES). Biomed J 43:293–304
Hsu M‑H, Kuo H‑C, Lin J‑J et al (2020) Therapeutic hypothermia for pediatric refractory status epilepticus May Ameliorate post-status epilepticus epilepsy. Biomed J 43:277–284
Illingworth MA, Hanrahan D, Anderson CE et al (2011) Elevated VGKC-complex antibodies in a boy with fever-induced refractory epileptic encephalopathy in school-age children (FIRES). Develop Med Child Neuro 53:1053–1057
Jun JS, Lee ST, Kim R et al (2018) Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol 84:940–945
Kessi M, Liu F, Zhan Y et al (2020) Efficacy of different treatment modalities for acute and chronic phases of the febrile infection-related epilepsy syndrome: a systematic review. Seizure 79:61–68
Koh S, Wirrell E, Vezzani A et al (2021) Proposal to optimize evaluation and treatment of febrile infection-related epilepsy syndrome (FIRES): a report from FIRES workshop. Epilepsia Open 6:62–72
Kothur K, Bandodkar S, Wienholt L et al (2019) Etiology is the key determinant of neuroinflammation in epilepsy: elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia 60:1678–1688
Kramer U, Chi CS, Lin KL et al (2011) Febrile infection–related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia 52:1956–1965
Lai YC, Muscal E, Wells E et al (2020) Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann Clin Transl Neurol 7:2467–2474
Lam S‑K, Lu W‑Y, Weng W‑C et al (2019) The short-term and long-term outcome of febrile infection-related epilepsy syndrome in children. Epilepsy Behav 95:117–123
Lee H‑F, Chi C‑S (2018) Febrile infection-related epilepsy syndrome (FIRES): therapeutic complications, long-term neurological and neuroimaging follow-up. Seizure 56:53–59
Levine JM, Ahsan N, Ho E et al (2020) Genetic acute necrotizing encephalopathy associated with RANBP2: clinical and therapeutic implications in pediatrics. Mult Scler Relat Disord 43:102194
Lim GY, Chen CL, Shih CWD (2021) Utility and safety of perampanel in pediatric FIRES and other drug-resistant epilepsies. Child Neurol Open 8:2329048
Lin WS, Hsu TR (2021) Hypothesis: Febrile infection-related epilepsy syndrome is a microglial NLRP3 inflammasome/IL‑1 axis-driven autoinflammatory syndrome. Clin Transl Immunol 10:e1299
Luo T, Wang Y, Lu G et al (2022) Vagus nerve stimulation for super-refractory status epilepticus in febrile infection–related epilepsy syndrome: a pediatric case report and literature review. Childs Nerv Syst 38:1401–1404
Lyon G, Dodge PR, Adams R (1961) The acute encephalopathies of obscure origin in infants and children. Brain 84:680–708
Nausch E, Schaffeldt L, Tautorat I et al (2022) New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES) of unknown aetiology: a comparison of the incomparable? Seizure 96(2002):18–21
Payne ET, Koh S, Wirrell EC (2020) Extinguishing febrile infection-related epilepsy syndrome: pipe dream or reality? In: Seminars in neurology. Thieme, Stuttgart, pp 263–272
Ritter LM, Nashef L (2021) New-onset refractory status epilepticus (NORSE). Pract Neurol 21:119–127
Rojo DC, Harvey AS, Iona X et al (2012) Febrile infection-related epilepsy syndrome is not caused by SCN1A mutations. Epilepsy Res 100:194–198
Sakuma H, Horino A, Kuki I (2020) Neurocritical care and target immunotherapy for febrile infection-related epilepsy syndrome. Biomed J 43:205–210
Sculier C, Gaspard N (2019) New onset refractory status epilepticus (NORSE). Seizure 68:72–78
Serino D, Santarone ME, Caputo D et al (2019) Febrile infection-related epilepsy syndrome (FIRES): prevalence, impact and management strategies. Neuropsychiatr Dis Treat 15:1897
Shorvon S, Ferlisi M (2012) The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain 135:2314–2328
Tan THL, Perucca P, O’brien TJ et al (2021) Inflammation, ictogenesis, and epileptogenesis: an exploration through human disease. Epilepsia 62:303–324
Van Baalen A, Häusler M, Boor R et al (2010) Febrile infection–related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia 51:1323–1328
Van Baalen A, Häusler M, Plecko-Startinig B et al (2012) Febrile infection-related epilepsy syndrome without detectable autoantibodies and response to immunotherapy: a case series and discussion of epileptogenesis in FIRES. Neuropediatrics 43:209–216
Van Baalen A, Vezzani A, Häusler M et al (2017) Febrile infection–related epilepsy syndrome: clinical review and hypotheses of epileptogenesis. Neuropediatrics 48:5–18
Yang JH, Nataraj S, Sattar S (2021) Successful treatment of pediatric FIRES with Anakinra. Pediatr Neurol 114:60–61
Funding
Open access funding provided by University of Basel
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Reppucci and A.N. Datta declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reppucci, D., Datta, A.N. FIRES—Pathophysiology, therapeutical approach, and outcome. Z. Epileptol. 35, 322–331 (2022). https://doi.org/10.1007/s10309-022-00533-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10309-022-00533-5