Abstract
Background
Status epilepticus (SE) is a common complication in patients surviving a cardiac arrest, but little is known about the frequency of nonconvulsive status epilepticus (NCSE).
Objectives
To compile the first the evidence from the literature of the overall frequency of NCSE in adults with persistent coma following cardiac arrest. Secondarily, to assess the emergence of NCSE in comatose resuscitated patients within the first hours of the return of spontaneous circulation (ROSC) and before inducing target temperature management.
Material and methods
The medical search engine PubMed was screened to identify prospective and retrospective studies in English reporting on the frequency of NCSE in comatose post-resuscitated patients. Study design, time of EEG performance, detection of SE and NCSE, outcomes, and targeted temperature management were assessed.
Results
Only three cohort studies (one prospective and two retrospective) reported on the EEG evaluation describing NCSE during ongoing sedation and target temperature management. Overall, we identified 213 patients with SE in 18–38% and NCSE in 5–12%. Our review found no study reporting NCSE in resuscitated adult patients remaining in coma within the first hours of ROSC and prior to targeted temperature management and sedation.
Conclusion
Studies of NCSE after ROSC in adults are rare and mostly nonsystematic. This and the low proportion of patients reported having NCSE following ROSC suggest that NCSE before target temperature management and sedation is often overlooked. The limited quality of the data does not allow firm conclusions to be drawn regarding the effects of NCSE on outcome calling for further investigations. Clinicians should suspect NCSE in patients with persistent coma before starting sedation and targeted temperature management.
Zusammenfassung
Hintergrund
Der Status epilepticus (SE) ist eine häufige Komplikation bei Patienten, die einen Herzstillstand überleben, aber über die Häufigkeit des nichtkonvulsiven Status epilepticus (NCSE) ist wenig bekannt.
Ziele
Zunächst erfolgt eine Darstellung der Nachweise aus der Literatur zur Häufigkeit von NCSE bei Erwachsenen mit persistierendem Koma nach einem Herz-Kreislauf-Stillstand. Danach wird das Auftreten von NCSE bei komatösen wiederbelebten Patienten innerhalb der ersten Stunde nach der Rückkehr des spontanen Kreislaufs (ROSC) und vor der Einleitung des Zieltemperaturmanagements beurteilt.
Material und Methoden
Die medizinische Datenbank PubMed wurde benutzt, um prospektive und retrospektive Studien in englischer Sprache zu identifizieren, die über die Häufigkeit von NCSE bei komatösen Patienten nach Reanimation berichten. Studiendesign, durchgeführte Elektroenzephalographien (EEG) und deren Befund, Erkennung von SE und NCSE, Ergebnisse und gezieltes Temperaturmanagement wurden bewertet.
Ergebnisse
Nur 3 Kohortenstudien (eine prospektive und 2 retrospektive) berichteten über eine EEG-Auswertung, die NCSE während der laufenden Sedierung und des Zieltemperaturmanagements beschrieb. Insgesamt wurden 213 Patienten mit SE in 18–38 % und NCSE in 5–12 % der Fälle erfasst. Es fand sich keine Studie, die über NCSE bei erwachsenen Reanimationspatienten berichtete, die innerhalb der ersten Stunden nach ROSC und vor gezieltem Temperaturmanagement und Sedierung im Koma blieben.
Schlussfolgerung
Studien zum NCSE nach ROSC bei Erwachsenen sind selten und meist unsystematisch. Dies und der geringe Anteil an Patienten, welche NCSE nach ROSC aufwiesen, legen nahe, dass NCSE vor Zieltemperaturmanagement und Sedierung oft übersehen wird. Die begrenzte Qualität der Daten lässt keine eindeutigen Schlussfolgerungen hinsichtlich der Auswirkungen von NCSE auf das Outcome zu, was weitere Untersuchungen erforderlich macht. Kliniker sollten bei Patienten mit persistierendem Koma einen NCSE vermuten, bevor sie mit einer Sedierung und einem gezielten Temperaturmanagement beginnen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac arrest is the most common cause of death, resulting in up to 60,000 annual deaths in out-of-hospital cardiac arrests in Switzerland [20]. The degree of hypoxic–ischemic brain injury and dysfunction varies and can result in persistent coma or death [6, 7, 11].
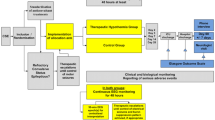
For patients with persistent coma following return of spontaneous circulation (ROSC), the recommendations for initial care include artificial ventilation, sedation, and neuroprotective measures including continuous monitoring of core temperature to detect and suppress fever (defined as a temperature > 37.7 °C) for at least 72 h [4, 12, 13]. In up to one third of these patients, seizures and status epilepticus (SE) may emerge with or without clinically overt signs [1, 16, 17, 19]. While recovery from post-cardiac arrest SE has rarely been described with good outcome [5, 8, 17, 19], it has been identified as an independent predictor of poor outcome in larger cohorts [16]. In contrast to convulsive SE, nonconvulsive epileptic complications [17, 18] can be frequently missed especially without continuous EEG (c-EEG) and little is known about the frequency and effects of nonconvulsive SE (NCSE) in this context. Although in the early treatment phase of resuscitated patients with persistent coma, detection of NCSE before the initiation of iso- or hypothermia is not part of most treatment protocols, it seems plausible that unrecognized and untreated ongoing SE may cause subsequent neurologic injury that has the potential to cause further cerebral damage that may be relevant.
We aimed to compile the current evidence from the literature on the emergence of NCSE in adults with persistent coma in particular within the first hours post-ROSC and before inducing target temperature management.
Methods
We screened PubMed using the predefined search terms “status epilepticus,” “nonconvulsive status epilepticus,” “continuous electroencephalogram,” “continuous EEG” in combination with the term “cardiac arrest,” “postanoxic,” “ischemic-anoxic.” Papers in a language other than English, papers not describing SE semiology, pediatric studies, and animal models were excluded. Prospective or retrospective studies and case series reporting the frequency of NCSE were included. Data regarding study design, number of patients, time of EEG performance, number of patients with SE, clinical signs of SE, proportion of patients with early NCSE, reported outcomes of patients with NCSE, and targeted temperature management were assessed.
Results
Detailed information of three identified prospective and retrospective cohort studies reporting the frequency of NCSE in the first hours during ongoing sedation and target temperature management is compiled in Table 1. Studies targeting explicitly the presence of NCSE in resuscitated adult patients with persistent coma after ROSC and prior to the initiation of targeted temperature management could not be identified by our review of the literature.
Overall, the three studies examined a total of 213 patients with SE in 18–38% and NCSE in 5–12% of patients.
The first and only study with a clear focus on NCSE in adult post-cardiac arrest patients was performed retrospectively and published by Rittenberger et al. in 2012 [15]. In that study, NCSE was detected in 12% of patients (12/101) using a 22-channel c‑EEG recording at a median of 9 h (range: 6–12 h) from cardiac arrest. Patients were already under targeted temperature management with a mean temperature of 33.9 °C and sedated for 48 h. Nonconvulsive status epilepticus was detected at the onset of the recording in three of the 12 (25%) patients and had a median duration of 5 h. The outcome was poor with only one patient (1%) surviving with a Cerebral Performance Category score (CPC) of 1.
Nonconvulsive status epilepticus was reported in two other studies on predictors of awakening from post-ischemic–hypoxic SE [17] and on frequency and timing of epileptiform activity on c‑EEG in comatose patients [10]. The first study (consisting of one large prospective and one retrospective part) comprising a total of 181 patients (with 74 patients investigated prospectively) revealed SE in 38% of the prospectively examined patients (28/74). The EEG was carried out using 14 or 21 channels. Data regarding EEG timing and duration of monitoring were not provided. The reported semiology was myoclonus in 31%, prolonged tonic–clonic seizures in 1%, and no motor symptoms in 5% [17]. While all but one of the patients having SE with motor symptoms died during their hospital stay, one patient remained in a minimally conscious state before dying in hospital and one significantly improved (i.e., CPC score of 2) [17].
Another retrospective study examined 38 comatose patients treated with therapeutic hypothermia and monitored with a 16–18 channel c‑EEG that was applied within a median of 15 h from arrest (range: 7.5–21.3 h) [10]. This monitoring detected SE in 23% of patients (7/38) with 13% (5/38) having SE with motors symptoms and 5% (2/38) being in NCSE. All these patients with NCSE died in hospital.
Discussion
Our review of the literature identified only three cohort studies of different designs, limited quality, and with very limited information regarding the emergence of early NCSE following cardiac arrest and associated outcomes.
Any studies on the emergence of NCSE in resuscitated adult patients remaining in coma within the first hours of ROSC and prior to the initiation of targeted temperature management were identified.
The fact that only very few data were identified linking the emergence of NCSE in the early stage of post-ROSC management to a specific presumed outcome seems unjustified and critical. In addition, the data compiled in this review do not allow any conclusions to be drawn on the effects of early detection and aggressive treatment of NCSE on outcome, nor on whether an early NCSE can be only a transient condition with a different clinical impact than SE occurring at a later stage.
With the exception of the retrospective study by Rittenberger and colleagues in 2012 that used a 22-channel continuous EEG recording early after cardiac arrest and focused on the frequency of NCSE [15], the studies did not perform EEGs in the early stage and were not primarily designed to investigate the emergence of NCSE in this context. Furthermore, the study of Rittenberg et al. [15] had limitations regarding the diagnosis of NCSE. Although the authors stated that SE was considered nonconvulsive if no motor symptoms were seen on the recorded video or at clinical examination, they had to admit that only a minority of individuals had such video recordings during EEG. Moreover, one NCSE patient was described as having subtle motor symptoms, not strictly fulfilling the criteria for NCSE.
Overall, the EEGs were rarely performed very early across studies (the earliest was at a median of 7 h), in very different time windows among the studies, and sometimes the time at which the EEG was started was not reported. Moreover, the fact that EEG, even if performed early, was always performed on patients already under hypothermia and ongoing sedation is a major limitation, as both hypothermia and sedatives may have antiseizure effects. Therapeutic hypothermia per se has some antiseizure effects [14] and often requires the administration of anesthetics, such as midazolam or propofol, which may further suppress seizures. Moreover, hypothermia can increase the blood concentration of propofol [9] and its anticonvulsant effects. To what degree established transient hypothermia and sedation in the context of targeted temperature management terminate seizures despite not have been titrated to a stable burst-suppression for 48 h, as recommended in cases of treatment-refractory SE by the International League Against Epilepsy (ILAE; [3]) remains unanswered. Hence, we cannot exclude that patients with missed early NCSE would benefit from supplemental antiseizure medication in addition to antiseizure effects from hypothermia and anesthetics regarding seizure termination and outcome. The concept of a “three-dimensional” biological continuum clearly shows the complexity in the interrelation between structural brain damage, epileptic activity, and the degree of coma, and how an excessive epileptic activity can play an important role in the worsening of the brain damage itself [2].
Conclusion
Studies on nonconvulsive status epilepticus (NCSE) immediately after return of spontaneous circulation (ROSC) in adults and prior to the initiation of targeted temperature management are lacking and the few studies on NCSE following ROSC are unsystematic and of low quality. These findings likely show that NCSE prior to targeted temperature management and sedation is often missed. Further studies are urgently needed to uncover the true frequency of NCSE and its effect on outcome in this context and to clarify whether rigorous initiation of aggressive antiseizure treatment may be key for better recovery. In the meantime, we suggest withdrawing sedation once patients are admitted to the intensive care unit and with persistent coma to start EEG monitoring to detect or exclude NCSE. This procedure, however, should be postponed in favor of the initially necessary and more vital support interventions. The complete neurological prognostication assessment should then strictly follow current international guidelines.
References
Amorim E, Rittenberger JC, Zheng JJ et al (2016) Continuous EEG monitoring enhances multimodal outcome prediction in hypoxic-ischemic brain injury. Resuscitation 109:121–126. https://doi.org/10.1016/j.resuscitation.2016.08.012
Bauer G, Trinka E (2010) Nonconvulsive status epilepticus and coma. Epilepsia 51:177–190. https://doi.org/10.1111/j.1528-1167.2009.02297.x
Brophy GM, Bell R, Claassen J et al (2012) Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 17:3–23. https://doi.org/10.1007/s12028-012-9695-z
Dankiewicz J, Cronberg T, Lilja G et al (2021) Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med 384:2283–2294. https://doi.org/10.1056/nejmoa2100591
Dragancea I, Backman S, Westhall E et al (2015) Outcome following postanoxic status epilepticus in patients with targeted temperature management after cardiac arrest. Epilepsy Behav 49:173–177. https://doi.org/10.1016/j.yebeh.2015.04.043
Gorjup V, Radsel P, Kocjancic ST et al (2007) Acute ST-elevation myocardial infarction after successful cardiopulmonary resuscitation. Resuscitation 72:379–385. https://doi.org/10.1016/j.resuscitation.2006.07.013
Hosmane VR, Mustafa NG, Reddy VK et al (2009) Survival and neurologic recovery in patients with ST-segment elevation myocardial infarction resuscitated from cardiac arrest. J Am Coll Cardiol 53:409–415. https://doi.org/10.1016/j.jacc.2008.08.076
Hovland A, Nielsen EW, Klüver J, Salvesen R (2006) EEG should be performed during induced hypothermia. Resuscitation 68:143–146. https://doi.org/10.1016/j.resuscitation.2005.05.019
Leslie K, Sessler DI, Bjorksten AR, Moayeri A (1995) Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg 80:1007–1014. https://doi.org/10.1097/00000539-199505000-00027
Mani R, Schmitt SE, Mazer M et al (2012) The frequency and timing of epileptiform activity on continuous electroencephalogram in comatose post-cardiac arrest syndrome patients treated with therapeutic hypothermia. Resuscitation 83:840–847. https://doi.org/10.1016/j.resuscitation.2012.02.015
Neumar RW, Nolan JP, Adrie C et al (2008) Post-cardiac arrest syndrome. Circulation 118:2452–2483. https://doi.org/10.1161/CIRCULATIONAHA.108.190652
Nolan JP, Sandroni C, Andersen LW et al (2022) ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Resuscitation 172:229–236. https://doi.org/10.1016/j.resuscitation.2022.01.009
Nolan JP, Sandroni C, Böttiger BW et al (2021) European resuscitation council and European society of intensive care medicine guidelines 2021: post-resuscitation care. Intensive Care Med 47:369–421. https://doi.org/10.1007/s00134-021-06368-4
Polderman KH (2008) Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 371:1955–1969. https://doi.org/10.1016/S0140-6736(08)60837-5
Rittenberger JC, Popescu A, Brenner RP et al (2012) Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care 16:114–122. https://doi.org/10.1007/s12028-011-9565-0
Rossetti AO, Logroscino G, Liaudet L et al (2007) Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 69:255–260. https://doi.org/10.1212/01.wnl.0000265819.36639.e0
Rossetti AO, Oddo M, Liaudet L, Kaplan PW (2009) Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology 72:744–749. https://doi.org/10.1212/01.wnl.0000343006.60851.62
Ruijter BJ, van Putten MJAM, Hofmeijer J (2015) Generalized epileptiform discharges in postanoxic encephalopathy: quantitative characterization in relation to outcome. Epilepsia 56:1845–1854. https://doi.org/10.1111/epi.13202
Rundgren M, Westhall E, Cronberg T et al (2010) Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med 38:1838–1844. https://doi.org/10.1097/CCM.0b013e3181eaa1e7
SAMS (Swiss acedemy of medical sciences) (2013) Decision on cardiopulmonary resuscitation
Funding
Open access funding provided by University of Geneva.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P. De Stefano, H. Quintard, M. Seeck, and R. Sutter declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Stefano, P., Quintard, H., Seeck, M. et al. Nonconvulsive status epilepticus following cardiac arrest—are we missing the beginning?. Z. Epileptol. 35, 297–302 (2022). https://doi.org/10.1007/s10309-022-00532-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10309-022-00532-6