Abstract
In the feed industry, β-glucosidase has been widely used in the conversion of inactive and bounded soybean isoflavones into active aglycones. However, the conversion is frequently inhibited by the high concentration of intestinal glucose in monogastric animals. In this study, a GH1 β-glucosidase (AsBG1) with high specific activity, thermostability and glucose tolerance (IC50 = 800 mM) was identified. It showed great glucose tolerance against substrates with hydrophobic aryl ligands (such as pNPG and soy isoflavones). Using soybean meal as the substrate, AsBG1 exhibited higher hydrolysis efficiency than the GH3 counterpart Bgl3A with or without the presence of glucose in the reaction system. Furthermore, it is the first time to find that the endogenous β-glucosidase of soybean meal, mostly belonging to GH3, plays a role in the hydrolysis of soybean isoflavones and is highly sensitive to glucose. These findings lead to a conclusion that the GH1 rather than GH3 β-glucosidase has prosperous application advantages in the conversion of soybean isoflavones in the feed industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean isoflavone, having a structure similar to estrogen, is approximately 1.2–4.2 g/kg in soybean meal feed [20]. There are three free isoflavone aglycones (daidzein, genistein and glycitein) and nine bounded isoflavone glycosides, and those bounded ones account for 95–97% of soybean isoflavone. The isoflavone aglycones have attracted considerable attention for their distinguished functions in promoting animal growth, improving feed utilization, improving reproductive ability, etc. [10, 14]. Thus, how to transform the bounded glycosides into free forms in the intestinal tract of monogastric animals is of economic and environmental importance [13, 26].
β-Glucosidase is a glycoside hydrolases (GH) that acts on the glycosidic bonds of carbohydrate moiety to release nonreducing terminal glycosyl residues, glycoside, and oligosaccharides [2, 3]. Based on the sequences of catalytic domains, β-glucosidases are grouped into GH 1, 3, 5, 9, 30 and 116 (http://www.cazy.org/) [4, 11], and most of them are confined in families 1 and 3. Based on the substrate specificity, β-glucosidases are grouped into aryl-β-glucosidase, true cellobiase, and β-glucosidase with broad substrate specificity [24, 30]. Those β-glucosidases acting on cellobiose and soybean isoflavones have important application values in the feed, food, biofuel and other fields [25, 32, 34]. One bottleneck of β-glucosidase application in the feed industry is its sensitivity to glucose, which is commonly assessed by measuring the half inhibitory concentration of glucose (IC50) or inhibition constant (Ki). Recent studies have shown that GH1 and GH3 β-glucosidases vary in their tolerance to glucose. In comparison to the GH3 β-glucosidases that have IC50 values of less than 100 mM [6, 16, 36] due to the competitive inhibition by glucose [5, 12, 31], the GH1 counterparts have an IC50 value of several hundreds to thousands of millimole [28, 33, 35]. A few studies have been carried out to reveal the high glucose tolerance mechanism of GH1 β-glucosidases. Giuseppe et al. compared the catalytic pocket structures of GH1 and GH3 β-glucosidases and found that the deeper catalytic pocket of GH1 β-glucosidases may account for their high glucose tolerance [9]; Yang et al. ascribed the high glucose tolerance of GH1 β-glucosidases to the substrate binding to multiple non-catalytic sites and their transglycosylation activities [40]. These studies gave some hints on the glucose tolerance mechanism of GH1 β-glucosidases [41], but no general accepted view was proposed.
In the intestinal tract of monogastric animals, the conversion efficiency of soybean isoflavones is low mainly due to the deficiency of β-glucosidase and the competitive inhibition of glucose [38]. One practice is to supplement exogenous β-glucosidase into soybean meal feed to increase the utilization of soy isoflavones [21, 29], the other is to screen and engineer the β-glucosidase with high glucose tolerance. The present study aims to obtain a β-glucosidase with great glucose tolerance and conversion efficiency of soybean isoflavones for potential application in the feed industry. A GH1 β-glucosidase (AsBG1) with high specific activity, thermostability and glucose tolerance (IC50= 800 mM) was identified from Alicyclobacillus sp. A4. Biochemical characterization showed that AsBG1 had glucose tolerance only against substrates with hydrophobic aryl ligands (such as pNPG and soy isoflavones). Further comparison of the hydrolysis capabilities of GH1 and GH3 β-glucosidases on soy isoflavones revealed that GH1 β-glucosidases have more application advantages and more prosperous potentials than GH3 ones in the feed industry.
Materials and methods
Strains, culture media, and reagents
The acidothermophilic Alicyclobacillus sp. A4 (whole genome sequenced) isolated from the hot spring water was stored in our laboratory [1]. GH3 β-glucosidase Bgl3A derived from Talaromyces leycettanus JCM12802 [376] was used as the reference. Ni2+-affinity beads from Suzhou Beaver Biomedical Engineering (China), and glucose oxidation (GOD-POD) kit from Beijing Leadman (China) were purchased. Substrates of p-nitrophenyl β-d-glucopyranoside (pNPG), p-nitrophenyl α-l-arabinofuranoside (pNPAf), p-nitrophenyl β-d-xylopyranoside (pNPX), barley β-glucan, lichenan, Avicel, and standard samples of daidzein, glycitein and genistein from Sigma-Aldrich (USA), cellobiose to cellohexose, laminaritetraose and maltose from Megazyme (Ireland), and daidzin from Tokyo Chemical Industry were obtained. The soybean meal was purchased from local market. All the other reagents were of analytical grade and commercially available.
Gene cloning and sequence analysis
Based on the genomic annotation of Alicyclobacillus sp. A4, a β-glucosidase gene of GH1, Asbg1, was identified. To obtain the full-length coding gene, a specific primer set Asbg1-F and Asbg1-R harboring 3 protection nucleotides, the restriction site of EcoRI or HindIII and 22 nucleotides of the 5′- and 3′-ends of Asbg1 (Table 1) were designed for the PCR amplification. The PCR products were purified, linked with vector pEASY-T3, and transformed into E. coli Trans1-T1 for sequencing.
Expression and purification
To produce the gene product in E. coli, four specific primers, Asbg1-pET30a-F and Asbg1-pET30a-R (each contained 20 bp of the pET30a sequence and 30 bp of the Asbgl sequence), and pET30a-Asbg1-F and pET30a-Asbg1-R (each contained 20 bp of the Asbgl sequence and 30 bp of the pET30a sequence) were designed (Table 1) to construct the recombinant expression plasmid pET30a-Asbg1. The target gene Asbg1 was ligated to the expression vector pET30a by overlap PCR method. The positive clones were verified by DNA sequencing, and then transformed into the E. coli BL21 (DE3) competent cells. The expression of recombinant AsBG1 was induced by isopropyl-β-d-thiogalactopyranoside (IPTG), and the crude enzyme was purified by Ni2+-affinity beads.
Assay of the enzymatic activity
One unit of enzymatic activity (U) was defined as the amount of β-glucosidase required to hydrolyze the substrate to produce 1 μmol of product per minute under certain reaction conditions. Each assay had triplicates. The assay methods for different substrates were described as below:
-
A.
pNP glycoside substrates. The reaction systems containing 250 μL of 4 mM pNP glycoside substrates and 250 μL of appropriately diluted enzyme solution were incubated at 55 °C and pH 6.5 for 10 min, followed by the addition of 1.5 mL of 1 M Na2CO3 to terminate the reaction. After cooling down to room temperature, the absorbance at OD405 was measured. The enzymatic activity was determined based on the amount of pNP released.
-
B.
Reducing oligosaccharide substrates. The GOD-POD method [22] was used. Reaction systems containing 70 μL of 4 mM oligosaccharide and 70 μL of appropriately diluted enzyme solution were incubated at 55 °C and pH 6.5 for 10 min, followed by 5 min-boiling water bath to terminate the reaction. After addition of 2.1 mL GOD reagent in the glucose oxidation kit, the reaction systems were incubated at 37 °C for 10 min. After cooling down to room temperature, the absorbance at OD520 was measured. The enzymatic activity was determined based on the amount of glucose released.
-
C.
Polysaccharide substrates. The DNS method [23] was used. The substrate solution, 900 μL of 0.5% (w/v) barley β-glucan, CMC-Na or lichenan, was preheated in a water bath for 2 min at 55 °C, followed by the addition of 100 μL of appropriately diluted enzyme solution. After incubation at 55 °C and pH 6.5 for 10 min, the reactions were terminated by adding 1.5 mL of DNS and boiling for 5 min. The OD540 values were then measured. The enzymatic activity was determined based on the amount of reducing sugar released.
Biochemical characterization
pNPG was used as the substrate for biochemical characterization of purified recombinant AsBG1. The optimal pH for AsBG1 was determined at 55 °C for 10 min in the buffers with pH 3.0–10.0. The optimal temperature was examined at the optimal pH 6.5 under the temperature range of 30‒80 °C. The pH stability was examined by measuring the residual enzyme activity under the standard conditions (pH 6.5 and 55 °C for 10 min) after pre-incubation of the enzyme without substrate in various pH buffer mentioned above for 1 h at 37 °C. And the thermostability was determined by measuring the residual activity under the standard conditions after the pre-incubation at 50 and 60 °C for 0, 5, 10, 20, 30, and 60 min without the substrate, respectively.
Substrate specificity and kinetic parameters
To scan the substrate spectrum, 2 mM of pNP materials (pNPG, pNPX and pNPAf), 5 mM of oligosaccharides (cellobiose to cellohexaose, laminaritetraose, and maltose), and 0.5‒1% (w/v) polysaccharides (lichenan, barley β-glucan, and Avicel) were used as substrates for AsBG1 activity assays under standard conditions (pH 6.5 and 55 °C for 10 min).
The initial reaction rate, V (μmol min mg−1), was determined under optimal conditions (pH 6.5 and 55 °C for 5 min) with 0.2‒1.5 mM pNPG or 1‒8 mM cellobiose as the substrate. A Lineweaver–Burk plot was drawn with the reciprocals of the substrate concentration (1/s, mM−1) and reaction rate (1/V, μmol−1 min−1 mg) as the X- and Y-axis, respectively. The substrate affinity, Km (mM) and the reaction velocity maximum, Vmax (μmol min mg−1), were calculated according to the formula 1/V = Km/Vmax × (1/s) + 1/Vmax, and the catalytic efficiency constant kcat/Km (s−1 mM−1) = Vmax× MW/60/Km, where MW represents the relative molecular mass (kDa) of AsBG1.
AsBG1 tolerance to monosaccharides and pNP
With pNPG as the substrate, the AsBG1 tolerance to different monosaccharides was determined. Reaction systems containing 125 μL of 8 mM pNPG, 250 μL of 0‒3 M monosaccharides (glucose, xylose, arabinose, galactose, fructose, and mannose), and 125 μL of appropriately diluted enzyme were incubated at 55 °C and pH 6.5 for 10 min, followed by the addition of 1.5 mL of 1 M Na2CO3 to terminate the reaction. The absorbance at OD405 was measured.
Glucose tolerance of AsBG1 with cellobiose as the substrate was also assessed. The reaction systems containing 100 μL of 8 mM cellobiose, 100 μL of glucose solution (at the final different concentration of 0, 40 or 80 mM) and 200 μL of the appropriately diluted enzyme solution were incubated at 55 °C and pH 6.5 for 10 min, followed by 5 min-boiling water bath. After cooling down to room temperature, the hydrolysis products were collected through a Vivaflow ultrafiltration membrane (Vivascience, Germany) with a molecular weight cut-off of 5 kDa, diluted 1000-fold with ddH2O, and analyzed by high performance ion exchange chromatography (HPIEC) with 1 μg mL−1 glucose as the standard. The substrate hydrolysis rate of AsBG1 was determined as follows: hydrolysis rate (%) = 100% × (Ac − At)/Ac, where Ac and At represent the substrate amounts determined in the blank (without enzyme addition) and treatment (with enzyme addition) samples, respectively.
The AsBG1 tolerance to pNP with pNPG and cellobiose as substrates was also assessed. The reaction systems consisted of 2 mM of pNPG or cellobiose, different concentrations of pNP (at the final concentration of 0, 10, 20, and 30 mM) and appropriately diluted enzyme solution.
Glucose tolerance against daidzin hydrolysis
Purified AsBG1 (GH1 family) is obtained from this study with the optimum pH 6.5 and Bgl3A (GH3 family) is available from our lab with the optimum pH 4.5 [37]. In this study, AsBG1 and Bgl3A were used as the comparative materials to hydrolysis daidzin. Reaction system containing 100 μL of 2 mM daidzin, 100 μL of 80 mM glucose and 200 μL of enzyme solution (0.036 U AsBG1 or Bgl3A) were incubated under the simulated intestinal conditions (37 °C and pH 6.5) for 5 min and terminated by a boiling water bath for 5 min. After cooling down to room temperature, the supernatants were collected by centrifugation at 12,000 rpm for 10 min, and then analyzed by HP1100 HPLC system (Waters, USA) equipped with a Diamonsil C18 column (5 μm × 250 mm × 4.6 mm; Dima, USA) with 2 mM daidzin as the standard. Reactions without enzyme or glucose addition were treated as controls. The conversion efficiency was determined based on the reduced amount of daidzin. Hydrolysis rate (%) = 100% × (Bc − Bt)/Bc, where Bc and Bt represent the amounts of daidzin in the control and treatment samples.
Conversion of soybean isoflavones under simulated intestinal conditions
Simulated soybean isoflavone hydrolysis by β-glucosidase AsBG1 or Bgl3A in the intestinal tract of monogastric animals was conducted with soybean meal as the substrate. Reaction systems containing 5 g crushed and sieved soybean meal and 15 mL buffer (containing 10 U enzyme and/or 10 mM glucose) were incubated at 37 °C at pH 6.5 for AsBG1 or pH 4.5 for Bgl3A in a water bath shaker for 2 h, followed by the addition of 35 mL of absolute ethanol and extraction of production at 65 °C for 2 h. The supernatants were then collected by high-speed centrifugation for HPLC analysis. Daidzein, glycitein and genistein were used as the standard samples for the quantitative analysis.
Results
Gene cloning and sequence analysis
The GH1 β-glucosidase-encoding gene, Asbg1 (GenBank accession no. KY039184), was obtained from the genome of Alicyclobacillus sp. A4. The full-length gene contained 1365 bp, and coded for 454 amino acid residues. Sequence analysis indicated that deduced AsBG1 had no putative signal peptide sequence and had a calculated molecular mass of 51.7 kDa. Multi-sequence alignment showed that AsBG1 had E166 and E354 as the catalytic residues.
Protein expression and purification
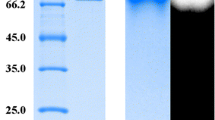
The recombinant plasmid pET30-Asbg1 was successfully constructed and transformed into E. coli BL21(DE3) competent cells for production. After IPTG induction and cell sonication, the cell lysate showed a β-glucosidase activity of approximately 100 U/mL. The crude enzyme was purified to homogeneity by Ni+ affinity beads. Purified recombinant AsBG1 migrated a single band of approximately 52 kDa in SDS-PAGE, which was consistent with the theoretical molecular weight (Fig. 1).
Biochemical characterization
Using pPNG as the substrate, purified recombinant AsBG1 displayed optimal activity at pH 6.5 and remained 70% of the maximal activity at pH 5.5–7.5 (Fig. 2a). Under the optimal pH, the enzyme exhibited the maximal activity at 55 °C, and remained highly active over the temperature range of 30‒60 °C (Fig. 2b). AsBG1 was stable over the acidic–alkaline range (pH 5.0–10.0), retaining more than 80% of the initial activity after 1-h incubation at 37 °C without substrate (Fig. 2c). The thermostability of AsBG1 was assayed. It retained highly stable after 1-h incubation at 50 °C, but lost up to 80% activity at 60 °C under same conditions (Fig. 2d).
Substrate specificity and kinetic parameters
The AsBG1 had a broad substrate spectrum including oligosaccharides of both β-(1,4)- and β-(1,3)-glycosidic linkages (Table 2) of the substrates tested, AsBG1 exhibited the highest activities against pNPG, cellooligosaccharides and laminaritetraose. The AsBG1 activities towards cellotriose, cellotetraose, cellopentose and cellohexaose were significantly higher, approximately twofold, than that to cellobiose. Besides, it had weak or detectable activities towards pNPX, pNPAf, maltose, and polysaccharides barley β-glucan, Avicel, and lichenan.
When using pNPG as the substrate, the purified recombinant AsBG1 had the Km, Vmax and kcat/Km values of 0.4 mM, 111 μmol min−1 mg−1, and 215 s−1 mM−1, respectively. The Km, Vmax and kcat/Km values of AsBG1 with cellobiose were 7.3 mM, 54 μmol min−1 mg−1, and 9 s−1 mM−1, respectively.
AsBG1 tolerance to monosaccharides and pNP
When using pNPG as the substrate, AsBG1 was highly tolerant to a variety of monosaccharides (Fig. 3). In the presence of 50‒200 mM glucose, AsBG1 showed enhanced activities up to 120%. Other tested oligosaccharides (including galactose, xylose, arabinose, galactose and fructose) at the concentration of ≤ 500 mM stimulated the AsBG1 activity by 1.2–1.8-fold. When increased the concentration to 3 M, arabinose and fructose still had stimulatory effects on AsBG1 activity.
The AsBG1 tolerance to pNP and glucose with pNPG and cellobiose as the substrate was also tested. When using pNPG as the substrate, AsBG1 had an IC50 value of up to 800 mM glucose (Fig. 4a). With cellobiose as the substrate, 10 and 20 mM glucose inhibited approximately 60 and 90% of enzyme activities (Fig. 4b). The product p-nitrophenol (pNP) strongly inhibited the AsBG1 activity. As shown in Fig. 4c, 10 mM pNP inhibited 40% of the enzyme activity. With cellobiose as the substrate, the presence of 10 mM pNP caused the 70% activity lost (Fig. 4d).
AsBG1 tolerance to glucose and pNP. a Glucose tolerance in the presence of 2 mM pNPG; b Glucose tolerance in the presence of 2 mM cellobiose; c pNP tolerance in the presence of 2 mM pNPG; d pNP tolerance in the presence of 2 mM cellobiose. Controls without addition of glucose or pNP were defined as 100%. Each value in the panel represents the mean ± SD (n = 3)
Glucose tolerance against daidzin hydrolysis
Under simulated intestinal conditions (10 mM glucose, 0.5 mM daidzin, 37 °C, pH 6.5, and 5 min), the hydrolysis rates of AsBG1 and Bgl3A, each at 0.036 U, were determined. Bgl3A converted 15 and 26% daidzin with and without glucose, while AsBG1 degraded 21 and 20% daidzin under the same conditions. The results indicated that the conversion efficiency of daidzin by Bgl3A was seriously impeded by glucose. Instead, the presence of glucose even stimulated the enzymatic activity of AsBG1 towards daidzin.
Simulated conversion of soybean isoflavones in intestinal tract
The conversion efficiencies of AsBG1 and Bgl3A towards soybean isoflavones were compared with and without glucose addition (Fig. 5a). Without enzyme addition, 22 mg L−1 daidzein, 4 mg L−1 glycitein and 24 mg L−1 genistein were detected in the reaction mixtures. When 10 U AsBG1 were added, the amounts of daidzein, glycitein and genistein released with and without glucose were increased to 71–79 mg L−1, 10–12 mg L−1, 104–118 mg L−1, which had no statistical differences. In contrast, the amounts of daidzein (38 mg L−1), glycitein (4 mg L−1) and genistein (38 mg L−1) released by Bgl3A were much less than those of AsBG1. The presence of 10 mM glucose further decreased the conversion efficiencies of Bgl3A by 9, 2, 12 mg L−1, and the ANOVA analysis showed significant differences between the two groups of with and without glucose addition of Bgl3A.
Based on the observed results above, the degradation of soybean meal by itself was further studied without adding exogenous enzyme solution (Fig. 5b). The results showed that the initial concentrations of daidzein, glycitein and genistein in the control group (reaction for 0 h) for both GH1 and GH3 were about 5, 2 and 6 mg L−1, respectively. After reaction time of 2 h, the production amount of daidzein, glycitein and genistein in the first reaction group (adding buffer only) for both GH1 and GH3 were increased to about 20, 4 and 24 mg L−1. After reaction time of 2 h, the production amount of daidzein in the second reaction group (adding buffer and 10 mM glucose) for both GH1 and GH3 was decreased about 33% lower than that of the first reaction group (adding buffer only).
Discussion
The genus Alicyclobacillus is well-known for its capability of producing various hydrolases. In a previous study, a β-glucosidase of GH1, Aaβ-gly (WP_008336965.1), has been identified in A. acidocaldarius and biochemically characterized [17]. Asbg1 under this study is the second known GH1 β-glucosidase from Alicyclobacillus. These two β-glucosidases shared 69% amino acid sequence identity and were both heterologously produced in E. coli. In comparison to the recombinant Aaβ-gly that has optimal activities at 65 °C, the purified recombinant AsBG1 has a temperature optimum of 55 °C and remains more active over the moderate temperature range (30‒60 °C). Therefore, the AsBG1 is more favorable for application in the food and feed industries.
AsBG1 was distinguished for its broad substrate specificity. It acted on both oligosaccharides (cellooligosaccharides and laminarioligosaccharides) and polysaccharides (barley β-glucan, lichenan, and Avicel). Of the five cellooligosaccharides tested, AsBG1 demonstrated much higher activities, approximately twofold, towards cellotriose to cellohexaose than to cellobiose. The results are contrary to the β-glucosidases derived from Aspergillus oryzae and Aspergillus niger with increased activities on cellobiose [27, 39]. The catalytic efficiencies of AsBG1 towards pNPG and cellobiose were 215 and 9 s−1 mM−1, respectively, which are much higher than the β-glucosidases from Humicola insolens and Trichoderma reesei [18, 33], but lower than Aaβ–gly [17]. The extensive substrate specificity and relatively high catalytic efficiency make AsBG1 potentially applicable in the feed, bioenergy, food, textile and other fields.
A few studies have shown that the glucose tolerance of GH1 β-glucosidase is much stronger than that of GH3 counterparts [7, 8, 15, 19]. Although great efforts have been exerted to analyze the glucose tolerance mechanism of GH1 β-glucosidases using molecular mutation techniques and molecular dynamics simulations [5, 18, 33], no general accepted view has been proposed so far. In the present study, AsBG1 showed great tolerance to a variety of monosaccharides (glucose, lactose, arabinose, galactose, fructose, and xylose) with pNPG as the substrate. Glucose as one of the most important product in many industrial processes showed stimulatory effects (up to 120%) on the AsBG1 activity at the concentration of 200 mM, with the IC50 value of up to 800 mM. However, when using cellobiose as the substrate, the enzyme showed sensitivity to both glucose and pNP. These results indicated that the high glucose tolerance of AsBG1 only functioned on aromatic substrate pNPG. The result of daidzin hydrolysis further demonstrated that the conversion efficiency of daidzin by Bgl3A (GH3) was seriously impeded by glucose while AsBG1 was not. These findings indicated the greater potential advantage of GH1 than GH3 β-glucosidases in the feed industry.
Soybean isoflavone, as an important component of corn soybean meal feed, plays an important role in promoting animal growth and improving feed conversion rate. The performance of AsBG1 hydrophobic aryl ligands and Bgl3A in the hydrolysis of soybean isoflavone in soybean meal was also compared. With three free, active aglycones (daidzein, glycitein, genistein) in soybean isoflavones as target products, the soybean meal was treated with or without GH1/3 β-glucosidase addition. The results showed that the hydrolysis capability of AsBG1 on soybean isoflavone was much stronger than that of Bgl3A, with the higher daidzein yield of 52%. With the presence of 10 mM glucose, the daidzein yield by AsBG1 hydrolysis reached 88% higher than that of Bgl3A. It is surprising to find that the daidzein yield of the reaction groups (with addition of enzyme and glucose) was 55% lower than that of the blank group (with buffer only). Further analysis revealed that the endogenous β-glucosidase contained in soybean meal might play a role in the hydrolysis. Sequence analysis on the endogenous β-glucosidase of soybean meal indicated that most of them belong to GH3, while those of GH1 frequently are mainly intracellular without signal peptide. These exogenous and endogenous β-glucosidases of GH3 might be inhibited by the glucose in the reaction system.
In summary, a neutral mesophilic β-glucosidase of GH1 with broad substrate specificity was identified in Alicyclobacillus sp. A4 and functionally verified in E. coli BL21. This study demonstrated, for the first time, that the GH1 β-glucosidase only showed glucose tolerance against substrates with hydrophobic aryl ligands (such as pNPG and soy isoflavones) but not substrates such as cellobiose. In the hydrolysis process of soybean isoflavone in soybean meal, GH1 exhibited better hydrolysis efficiency than GH3 β-glucosidase with or without glucose addition. Besides, it is also the first time to find that the endogenous β-glucosidase contained in soybean meal plays a role in the hydrolysis of soybean isoflavone to some extent and is strongly inhibited by glucose. All of these findings showed that GH1 rather than GH3 β-glucosidases have more application advantages in the conversion of soybean isoflavones in the feed industry.
References
Bai Y, Wang J, Zhang Z, Shi P, Luo H, Huang H, Feng Y, Yao B (2010) Extremely acidic β-1,4-glucanase, CelA4, from thermoacidophilic Alicyclobacillus sp. A4 with high protease resistance and potential as a pig feed additive. J Agric Food Chem 58:1970–1975
Bhatia Y, Mishra S, Bisaria VS (2002) Microbial β-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol 22:375–407
Cairns JRK, Esen A (2010) β-Glucosidases. Cell Mol Life Sci 67:3389–3405
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active enzymes database (Cazy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Cao L, Wang Z, Ren G, Kong W, Li L, Xie W, Liu Y (2015) Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol Biofuels 8:202–209
Chauve M, Mathis H, Huc D, Casanave D, Monot F, Ferreira NL (2010) Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol Biofuel 3:1–8
Chen K, Arnold FH (1993) Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide. Proc Nat Acad Sci 90:5618–5622
Decker CH, Visser J, Schreier P (2000) β-Glucosidases from five black Aspergillus species: study of their physico-chemical and biocatalytic properties. J Agric Food Chem 48:4929–4936
de Giuseppe PO, Souza Tde A, Souza FH, Zanphorlin LM, Machado CB, Ward RJ, Jorge JA, Furriel Rdos P, Murakami MT (2014) Structural basis of glucose tolerance in GH1 & #x03B2;-glucosidase. Acta Cryst D70:1631–1639
Goodmangruen D, Kritzsilverstein D (2001) Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr 131:1202–1206
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644
Hsieh CC, Cannella D, Jørgensen H, Felby C, Thygesen LG (2014) Cellulase inhibition by high concentrations of monosaccharides. J Agric Food Chem 62:3800–3804
Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M (2000) Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 130:1695–1699
Kawakami Y, Tsurugasaki W, Nakamura S, Osada K (2005) Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J Nutr Biochem 16:205–212
Korotkova OG, Semenova MV, Morozova VV (2009) Isolation and properties of fungal β-glucosidases. Biochem Biokhimiia 74:569–577
Langston J, Sheehy N, Xu F (2006) Substrate specificity of Aspergillus oryzae family 3 β-glucosidase. Biochim Et Biophys Acta 1764:972–978
Lauro BD, Rossi M, Moracci M (2008) Characterization of a β-glycosidase from the thermoacidophilic bacterium Alicyclobacillus acidocaldarius. Extremophiles 12:309–320
Lee HL, Chang CK, Jeng WY (2012) Mutations in the substrate entrance region of β-glucosidase from Trichoderma reesei improve enzyme activity and thermostability. Prot Eng Des Sel Peds 25:733–738
Lu J, Du L, Wei Y, Hu Y, Huang R (2013) Expression and characterization of a novel highly glucose-tolerant β-glucosidase from a soil metagenome. Acta Biochim Et Biophys Sin 45:664–668
Luo H, Liu P, Yan-Dan LI (2005) Extraction of isoflavones aglycone from soybean. J Zhejiang Norm Univ 39:45–47
Mazur W, Wahala K, Wang GJ (1998) Dietary phytoestrogens from chemistry to chemoprevention. Kemia-kemi 25:48–54
Miksch R, Wiedemann G (1973) Blood sugar determination with the GOD-POD-ABTS method using uranyl acetate for deproteinization. Z Med Labortech 14:27–33
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Park TH, Choi KW, Park CS, Lee SB (2005) Substrate specificity and transglycosylation catalyzed by a thermostable β-glucosidase from marine hyperthermophile Thermotoga neapolitana. Appl Microbiol Biotechnol 56:411–422
Pogorzelski E, Wilkowska A (2010) Flavour enhancement through the enzymatic hydrolysis of glycosidic aroma precursors in juices and wine beverages: a review. Flavour Frag J 22:251–254
Qian LC, Sun JY (2009) Effect of β-glucosidase as a feed supplementary on the growth performance, digestive enzymes and physiology of broilers. Asian Aust J Anim Sci 22:260–266
Riou C, Salmon JM, Vallier MJ (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64:3607–3614
Rosa JC, Masui DC, Leone FA, Jorge JA (2010) Purification and biochemical characterization of a mycelial glucose- and xylose-stimulated β-glucosidase from the thermophilic fungus Humicola insolens. Process Biochem 45:272–278
Setchell KD (2000) Absorption and metabolism of soy isoflavones from food to dietary supplements and adults to infants. J Nutr 130:654S–655S
Singh G, Verma AK, Kumar V (2016) Catalytic properties, functional attributes and industrial applications of β-glucosidases. Biotechnology 6:1–14
Sinnott ML (1997) Enzymatic degradation of insoluble carbohydrates. Carbohydr Res 302:119–122
Song X, Xue Y, Wang Q, Wu X (2011) Comparison of three thermostable β-glucosidases for application in the hydrolysis of soybean isoflavone glycosides. J Agric Food Chem 59:1954–1961
Souza FHM, Inocentes RF, Ward RJ (2013) Glucose and xylose stimulation of a β-glucosidase from the thermophilic fungus Humicola insolens: a kinetic and biophysical study. J Mol Catal B Enzym 94:119–128
Sun H, Xue Y, Lin Y (2014) Enhanced catalytic efficiency in quercetin-4′-glucoside hydrolysis of Thermotoga maritima β-glucosidase A by site-directed mutagenesis. J Agric Food Chem 62:6763–6770
Thongpoo P, Srisomsap C, Chokchaichamnankit D, Kitpreechavanich V, Svasti J, Kongsaeree PT (2014) Purification and characterization of three β-glycosidases exhibiting high glucose tolerance from Aspergillus niger ASKU28. Biosci Biotechnol Biochem 78:1167–1176
Xia W, Bai Y, Cui Y, Xu X, Qian L, Shi P, Zhang W, Luo H, Zhan X, Yao B (2016) Functional diversity of family 3 β-glucosidases from thermophilic cellulolytic fungus Humicola insolens. Sci Rep 6:27062–27066
Xia W, Xu X, Qian L, Shi P, Bai Y, Luo H, Ma R, Yao B (2016) Engineering a highly active thermophilic β-glucosidase to enhance its pH stability and saccharification performance. Biotechnol Biofuels 9:147–152
Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S (1995) Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr 125:2307–2315
Yan TR, Lin CL (1997) Purification and characterization of a glucose-tolerant β-glucosidase from Aspergillus niger CCRC 31494. Biosci Biotechnol Biochem 61:965–970
Yang Y, Zhang X, Yin Q, Fang W, Fang Z, Wang X, Zhang X, Xiao Y (2015) A mechanism of glucose tolerance and stimulation of GH1 & #x03B2;-glucosidases. Sci Rep 5:17296
Zhang C, Wang X, Zhang W, Zhao Y, Lu X (2017) Expression and characterization of a glucose-tolerant-1,4-glucosidase with wide substrate specificity from Cytophaga hutchinsonii. Appl Microbiol Biotechnol 101:1919–1926
Acknowledgements
This research was supported by the National Special Program for GMO Development of China (Grant No.2016ZX08003-002), the Chinese Academy of Agricultural Science and Technology Innovation Project (CAAS-XTCX2016011-01) and the National Chicken Industry Technology System of China (CARS-41).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cao, H., Zhang, Y., Shi, P. et al. A highly glucose-tolerant GH1 β-glucosidase with greater conversion rate of soybean isoflavones in monogastric animals. J Ind Microbiol Biotechnol 45, 369–378 (2018). https://doi.org/10.1007/s10295-018-2040-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2040-6