Abstract
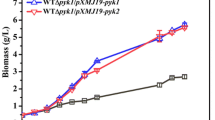
Corynebacterium glutamicum strains NC-2 were able to grow on xylose as sole carbon sources in our previous work. Nevertheless, it exhibited the major shortcoming that the xylose consumption was repressed in the presence of glucose. So far, regarding C. glutamicum, there are a number of reports on ptsG gene, the glucose-specific transporter, involved in glucose metabolism. Recently, we found ptsG had influence on xylose utilization and investigated the ptsG gene in response to xylose utilization in C. glutamicum with the aim to improve xylose consumption and simultaneously utilized glucose and xylose. The ptsG-deficient mutant could grow on xylose, while exhibiting noticeably reduced growth on xylose as sole carbon source. A mutant deficient in ptsH, a general PTS gene, exhibited a similar phenomenon. When complementing ptsG gene, the mutant ΔptsG-ptsG restored the ability to grow on xylose similarly to NC-2. These indicate that ptsG gene is not only essential for metabolism on glucose but also important in xylose utilization. A ptsG-overexpressing recombinant strain could not accelerate glucose or xylose metabolism. When strains were aerobically cultured in a sugar mixture of glucose and xylose, glucose and xylose could not be utilized simultaneously. Interestingly, the ΔptsG strain could co-utilize glucose and xylose under oxygen-deprived conditions, though the consumption rate of glucose and xylose dramatically declined. It was the first report of ptsG gene in response to xylose utilization in C. glutamicum.






Similar content being viewed by others
References
Aduse-Opoku J, Mitchell W (1988) Diauxic growth of Clostridium thermosaccharolyticum on glucose and xylose. FEMS Microbiol Lett 50(1):45–49
Aristidou A, Penttila M (2000) Metabolic engineering applications to renewable resource utilization. Curr Opin Biotechnol 11(2):187–198
Beall DS, Ohta K, Ingram LO (1991) Parametric studies of ethanol production form xylose and other sugars by recombinant Escherichia coli. Biotechnol Bioeng 38(3):296–303
Brückner R, Titgemeyer F (2002) Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209(2):141–148
Buschke N, Becker J, Schäfer R, Kiefer P, Biedendieck R, Wittmann C (2013) Systems metabolic engineering of xylose-utilizing Corynebacterium glutamicum for production of 1,5-diaminopentane. Biotechnol J 8(5):557–570
Buschke N, Schröder H, Wittmann C (2011) Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose. Biotechnol J 6(3):306–317
Chaillou S, Pouwels PH, Postma PW (1999) Transport of D-xylose in Lactobacillus pentosus, Lactobacillus casei, and Lactobacillus plantarum: evidence for a mechanism of facilitated diffusion via the phosphoenolpyruvate: mannose phosphotransferase system. J Bacteriol 181:4768–4773
Chatterjee R, Millard CS, Champion K, Clark DP, Donnelly MI (2001) Mutation of the ptsG gene resulted in increased production of succinate in fermentation of glucose by Escherichia coli. Appl Environ Microbiol 67(1):148–154
Chiang CJ, Lee HM, Guo HJ, Wang ZW, Lin LJ, Chao YP (2013) Systematic approach to engineer Escherichia coli pathways for co-utilization of a glucose–xylose mixture. J Agric Food Chem 61(31):7583–7590
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104(1–3):155–172
Hernández-Montalvo V, Valle F, Bolivar F, Gosset G (2001) Characterization of sugar mixtures utilization by an Escherichia coli mutant devoid of the phosphotransferase system. Appl Microbiol Biotechnol 57(1–2):186–191
Ikeda M, Mizuno Y, Awane S, Hayashi M, Mitsuhashi S, Takeno S (2011) Identification and application of a different glucose uptake system that functions as an alternative to the phosphotransferase system in Corynebacterium glutamicum. Appl Microbiol Biotechnol 90:1443–1451
Inui M, Kawaguchi H, Murakami S, Alain AV, Yukawa H (2004) Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol 8(4):243–254
Jakoby M, Ngouoto-Nkili CE, Burkovski A (1999) Construction and application of new Corynebacterium glutamicum vectors. Biotechnol Tech 13:437–441
Kawaguchi H, Vertès AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72(5):3418–3428
Kotrba P, Inui M, Yukawa H (2001) The ptsI gene encoding enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem Biophys Res Commun 289(5):1307–1313
Krings E, Krumbach K, Bathe B, Kelle R, Wendisch VF, Sahm H, Eggeling L (2006) Characterization of myo-inositol utilization by Corynebacterium glutamicum: the stimulon, identification of transporters, and influence on l-lysine formation. J Bacteriol 188(23):8054–8061
Lindner SN, Knebel S, Pallerla SR, Schoberth SM, Wendisch VF (2010) Cg2091 encodes a polyphosphate/ATP-dependent glucokinase of Corynebacterium glutamicum. Appl Microbiol Biotechnol 87(2):703–713
Lindner SN, Seibold GM, Henrich A, Krämer R, Wendisch VF (2011) Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases. Appl Environ Microbiol 77(11):3571–3581
Meiswinkel TM, Gopinath V, Lindner SN, Nampoothiri KM, Wendisch VF (2013) Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb Biotechnol 6(2):131–140
Moon MW, Park SY, Choi SK, Lee JK (2007) The phosphotransferase system of Corynebacterium glutamicum: features of sugar transport and carbon regulation. J Mol Microbiol Biotechnol 12(1–2):43–50
Nichols NN, Dien BS, Bothast RJ (2001) Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Appl Microbiol Biotechnol 56(1–2):120–125
Oh H, Park Y, Park C (1999) A mutated PtsG, the glucose transporter, allows uptake of d-ribose. J Biol Chem 274(20):14006–14011
Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H (2008) An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol 81(3):459–464
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68(4):475–480
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008) Production of d-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 78(3):449–454
Parche S, Burkovski A, Sprenger GA, Weil B, Krämer R, Titgemeyer F (2001) Corynebacterium glutamicum: a dissection of the PTS. J Mol Microbiol Biotechnol 3(3):423–428
Plumbridge J (1998) Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol Microbiol 27(2):369–380
Postma PW (1976) Involvement of the phosphotransferase system in galactose transport in Salmonella typhimurium. FEBS Lett 61:49–53
Postma PW, Lengeler JW (1985) Phosphoenolpyruvate: carbohydrate phosphotransfease system of bacteria. Microbiol Rev 49(3):232–269
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sasaki M, Jojima T, Inui M, Yukawa H (2010) Xylitol production by recombinant Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 86(4):1057–1066
Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H (2009) Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl Microbiol Biotechnol 85(1):105–115
Sasaki M, Teramoto H, Inui M, Yukawa H (2011) Identification of mannose uptake and catabolism genes in Corynebacterium glutamicum and genetic engineering for simultaneous utilization of mannose and glucose. Appl Microbiol Biotechnol 89(6):1905–1916
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
ter Kuile BH, Cook M (1994) The kinetics of facilitated diffusion followed by enzymatic conversion of the substrate. Biochim Biophys Acta 1193(2):235–239
Tao H, Gonzalez R, Martinez A, Rodriguez M, Ingram LO, Preston JF, Shanmugam KT (2001) Engineering a homo-ethanol pathway in Escherichia coli: increased glycolytic flux and levels of expression of glycolytic genes during xylose fermentation. J Bacteriol 183(10):2979–2988
Uhde A, Youn JW, Maeda T, Clermont L, Matano C, Krämer R, Wendisch VF, Seibold GM, Marin K (2013) Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum. Appl Microbiol Biotechnol 97(4):1679–1687
Van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52(4):541–545
Wang C, Zhang HL, Cai H, Zhou ZH, Chen YL, Chen YL, Ouyang PK (2013) Succinic acid production from corn cob hydrolysates by genetically engineered Corynebacterium glutamicum. Appl Biochem Biotechnol. doi:10.1007/s12010-013-0539-x
Wyman CE (1999) Production of low cost sugars from biomass: progress, opportunities, and challenges. In: Overend RP, Chornet E (eds) Biomass: a growth opportunity in green energy and valueadded products, vol 1. Pergamon Press, Oxford, pp 867–872
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56(1–2):17–34
Acknowledgments
This work was supported by the 973 Program of China (Grant No. 2011CB707405).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Cai, H., Zhou, Z. et al. Investigation of ptsG gene in response to xylose utilization in Corynebacterium glutamicum . J Ind Microbiol Biotechnol 41, 1249–1258 (2014). https://doi.org/10.1007/s10295-014-1455-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1455-y