Abstract
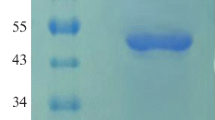
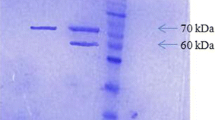
The phytase (PPHY) of Pichia anomala has the requisite properties of thermostability and acidstability, broad substrate spectrum, and protease insensitivity, which make it a suitable candidate as a feed and food additive. The 1,389-bp PPHY gene was amplified from P. anomala genomic DNA, cloned in pPICZαA, and expressed extracellularly in P. pastoris X33. Three copies of PPHY have been detected integrated into the chromosomal DNA of the recombinant P. pastoris. The size exclusion chromatography followed by electrophoresis of the pure rPPHY confirmed that this is a homohexameric glycoprotein of ~420 kDa with a 24.3 % portion as N-linked glycans. The temperature and pH optima of rPPHY are 60 °C and 4.0, similar to the endogenous enzyme. The kinetic characteristics K m, V max, K cat, and K cat/K m of rPPHY are 0.2 ± 0.03 mM, 78.2 ± 1.43 nmol mg−1 s−1, 65,655 ± 10.92 s−1, and 328.3 ± 3.12 μM−1 s−1, respectively. The optimization of medium components led to a 21.8-fold improvement in rPPHY production over the endogenous yeast. The rPPHY titer attained in shake flasks could also be sustained in the laboratory fermenter. The rPPHY accounts for 57.1 % of the total secreted protein into the medium. The enzyme has been found useful in fractionating allergenic protein glycinin from soya protein besides dephytinization.






Similar content being viewed by others
References
Arjmand S, Lotfi AS, Shamsara M, Mowla SJ (2013) Elevating the expression level of biologically active recombinant human alpha 1-antitrypsin in Pichia pastoris. Electron J Biotechnol. doi:10.2225/vol16-issue1-fulltext-4
Bai J, Swartz DJ, Protasevich II, Brouillette CG, Harrell PM, Hildebrandt E, Gasser B, Mattanovich D, Ward A, Chang G, Urbatsch IL (2011) A gene optimization strategy that enhances production of fully functional P-glycoprotein in Pichia pastoris. PLoS ONE 6(8):e22577
Chen BHY, Morr CV (1985) Solubility and foaming properties of phytate-reduced soy protein isolate. J Food Sci 50:1139–1142
Cregg JM, Tolstorukov I, Kusari A, Sunga J, Madden K, Chappell T (2009) Expression in the yeast, Pichia pastoris. Methods Enzymol 463:69–189
Harland BF, Morris ER (1995) Phytate: a good or bad food component. Nutr Res 15:733–754
Heinonen JK, Lahti RJ (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317
Holzhauser T, Wackermann O, Ballmer-Weber BK, Bindslev-Jensen C, Scibilia J, Perono-Garoffo L, Utsumi S, Poulsen LK, Vieths S (2009) Soybean (Glycine max) allergy in Europe: Gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J Allergy Clin Immunol 123(2):452–458
Inan M, Aryasomayajula D, Sinha J, Meagher MM (2005) Enhancement of protein secretion in Pichia pastoris by over expression of protein disulfide isomerase. Biotechnol Bioeng 93:771–778
Kaur P, Kunze G, Satyanarayana T (2007) Yeast phytase: present scenario and future perspectives. Crit Rev Biotechnol 27:93–109
Kaur P, Satyanarayana T (2005) Production of cell-bound phytase by Pichia anomala in an economical cane molasses medium: optimization using statistical tools. Process Biochem 40:3095–3102
Kaur P, Satyanarayana T (2010) Improvement in cell-bound phytase activity of Pichia anomala by permeabilization and applicability of permeabilized cells in soymilk dephytinization. J Appl Microbiol 108:2041–2049
Kobayashi K, Kuwae S, Ohya T, Ohda T, Ohyama M, Tomomitsu K (2000) Addition of oleic acid increases expression of recombinant human serum albumin by the AOX2 promoter in Pichia pastoris. J Biosci Bioeng 89(5):479–484
Leach BS, Collawan JF Jr, Fish WW (1980) Behaviour of glycopolypeptides with empirical molecular weight estimation methods. 1. Sodium dodecyl sulphate. Biochemistry 19:5734–5741
Ledeboer AM, Edens L, Maat J, Visser C, Bos JZ, Verrips CT, Janowicz Z, Eckart M, Roggenkamp R, Hollenberg CP (1985) Molecular cloning and characterization of a gene coding for methanol oxidase in Hansenula polymorpha. Nucleic Acids Res 13:3063–3082
Mitchell DB, Vogel K, Weimann BJ, Pasamontes L, van Loon AP (1997) The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiol 143:245–252
Pereira GG, Hollenberg CP (1996) Conserved regulation of the Hansenula polymorpha MOX promoter in Saccharomyces cerevisiae reveals insights in the transcriptional activation by Adr1p. Eur J Biochem 238:181–191
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 37:305–325
Prielhofer P, Maurer M, Klein J, Wenger J, Kiziak C, Gasser B, Mattanovich D (2013) Induction without methanol: novel regulated promoters enable high-level expression in Pichia pastoris. Microb Cell Fact 12:5
Ragon M, Neugnot-Roux V, Chemardin P, Moulin G, Boze H (2008) Molecular gene cloning and over expression of the phytase from Debaryomyces castellii CBS 2923. Protein Expr Purif 58:275–283
Sambrook J, Russell DW (2006) Southern blotting: capillary transfer of DNA to membranes. Cold Spring Harbor Protocols pdb.prot4040-pdb.prot4040
Satio T, Kohno M, Tsumura K, Kugimiya W, Kito M (2001) Novel method using phytase for separating soyabean β-conglycinin and glycinin. Biosci Biotechnol Biochem 65(4):884–887
Sicherer SH, Sampson HA (2006) Food allergy. J Allergy Clin Immunol 117(2):470–475
Singh B, Satyanarayana T (2013) Characterization of a HAP-phytase from a thermophilic mould Sporotrichum thermophile. Biores Technol 100:2046–2051
Stahl CH, Han YM, Roneker KR, House WA, Lei XG (1999) Phytase improves iron bioavailability for haemoglobin synthesis in young pigs. J Anim Sci 77:2135–2142
Tajoddin MD, Shinde M, Lalitha J (2011) In vivo reduction the phytic acid content of Mung Bean (Phaseolus aureus L) cultivars during germination. J Agric Environ Sci 10(1):127–132
Vats P, Banerjee UC (2005) Biochemical characterisation of extracellular phytase (myo-inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. J Ind Microbiol Biotechnol 32:141–147
Verivouri H, Seigler KM, Sands JA, Montenecourt BS (1985) Regulation of cellulose biosynthesis and secretion in fungi. Biochem Soc Trans 13:411–414
Verma D, Satyanarayana T (2012) Phytase production by the unconventional yeast Pichia anomala in fed-batch and cyclic fed-batch fermentations. Afr J Biotechnol 11:13705–13709
Vohra A, Satyanarayana T (2002) Purification and characterization of a thermostable and acid-stable phytase from Pichia anomala. World J Microbiol Biotechnol 18:687–691
Vohra A, Satyanarayana T (2003) Phytases: microbial sources, production, purification, and potential biotechnological applications. Crit Rev Biotechnol 23:29–60
Wang Y, Wang Z, Du G, Hu Z, Liu L, Li J, Chen J (2009) Enhancement of alkaline polygalacturonate lyase production in recombinant Pichia pastoris according to the ratio of methanol to cell concentration. Biores Techol 100:1343–1349
Woo JH, Liu YY, Neville DM Jr (2006) Minimization of aggregation of secreted bivalent anti-human T cell immunotoxin in Pichia pastoris bioreactor culture by optimizing culture conditions for protein secretion. J Biotechnol 121:75–85
Xuan NT, Hang MT, Thanh VN (2009) Cloning and over expression of an Aspergillus niger XP Phytase Gene (phyA) in Pichia pastoris. WASET 56:750–753
Yu P, Yan Y, Tang YP (2011) Medium optimization for endochitinase production by recombinant Pichia pastoris ZJGSU02 using response surface methodology. Afr J Biotechnol 10(1):75–84
Acknowledgments
We wish to thank University Grants Commission (UGC) and Council of Scientific & Industrial Research (CSIR), Govt. of India, New Delhi, for the financial assistance and awarding a fellowship to SJ, while carrying out this investigation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joshi, S., Satyanarayana, T. Optimization of heterologous expression of the phytase (PPHY) of Pichia anomala in P. pastoris and its applicability in fractionating allergenic glycinin from soy protein. J Ind Microbiol Biotechnol 41, 977–987 (2014). https://doi.org/10.1007/s10295-014-1407-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1407-6