Abstract
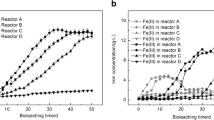
In order to better understand the bioleaching mechanism, expression of genes involved in energy conservation and community structure of free and attached acidophilic bacteria in chalcopyrite bioleaching were investigated. Using quantitative real-time PCR, we studied the expression of genes involved in energy conservation in free and attached Acidithiobacillus ferrooxidans during bioleaching of chalcopyrite. Sulfur oxidation genes of attached A. ferrooxidans were up-regulated while ferrous iron oxidation genes were down-regulated compared with free A. ferrooxidans in the solution. The up-regulation may be induced by elemental sulfur on the mineral surface. This conclusion was supported by the results of HPLC analysis. Sulfur-oxidizing Acidithiobacillus thiooxidans and ferrous-oxidizing Leptospirillum ferrooxidans were the members of the mixed culture in chalcopyrite bioleaching. Study of the community structure of free and attached bacteria showed that A. thiooxidans dominated the attached bacteria while L. ferrooxidans dominated the free bacteria. With respect to available energy sources during bioleaching of chalcopyrite, sulfur-oxidizers tend to be on the mineral surfaces whereas ferrous iron-oxidizers tend to be suspended in the aqueous phase. Taken together, these results indicate that the main role of attached acidophilic bacteria was to oxidize elemental sulfur and dissolution of chalcopyrite involved chiefly an indirect bioleaching mechanism.





Similar content being viewed by others
References
Afzal Ghauri M, Okibe N, Barrie Johnson D (2007) Attachment of acidophilic bacteria to solid surfaces: the significance of species and strain variations. Hydrometallurgy 85(2–4):72–80. doi:10.1016/j.hydromet.2006.03.016
Appia-Ayme C, Guiliani N, Ratouchniak J, Bonnefoy V (1999) Characterization of an operon encoding two c-type cytochromes, an aa3-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl Environ Microbiol 65(11):4781–4787
Beard S, Paradela A, Albar JP, Jerez CA (2011) Growth of Acidithiobacillus ferrooxidans ATCC 23270 in thiosulfate under oxygen-limiting conditions generates extracellular sulfur globules by means of a secreted tetrathionate hydrolase. Frontiers in microbiology 2. doi:10.3389/fmicb.2011.00079
Bengrine A, Guiliani N, Appia-Ayme C, Jedlicki E, Holmes DS, Chippaux M, Bonnefoy V (1998) Sequence and expression of the rusticyanin structural gene from Thiobacillus ferrooxidans ATCC33020 strain. BBA-Gene Struct Expr 1443(1–2):99–112
Brierley JA (1990) Acidophilic thermophilic archaebacteria: potential application for metals recovery. FEMS Microbiol Lett 75(2–3):287–291. doi:10.1016/0378-1097(90)90539-3
Crundwell FK (2003) How do bacteria interact with minerals? Hydrometallurgy 71(1–2):75–81
Gehrke T, Telegdi J, Thierry D, Sand W (1998) Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl Environ Microbiol 64(7):2743–2747
Harneit K, Göksel A, Kock D, Klock JH, Gehrke T, Sand W (2006) Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 83(1–4):245–254. doi:10.1016/j.hydromet.2006.03.044
He Z, Zhao J, Gao F, Hu Y, Qiu G (2010) Monitoring bacterial community shifts in bioleaching of Ni–Cu sulfide. Bioresour Technol 101(21):8287–8293. doi:10.1016/j.biortech.2010.05.047
Iwamoto T, Tani K, Nakamura K, Suzuki Y, Kitagawa M, Eguchi M, Nasu M (2000) Monitoring impact of in situ biostimulation treatment on groundwater bacterial community by DGGE. FEMS Microbiol Ecol 32(2):129–141. doi:10.1111/j.1574-6941.2000.tb00707.x
Kanao T, Kamimura K, Sugio T (2007) Identification of a gene encoding a tetrathionate hydrolase in Acidithiobacillus ferrooxidans. J Biotechnol 132(1):16–22
Klauber C, Parker A, van Bronswijk W, Watling H (2001) Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int J Miner Process 62(1–4):65–94. doi:10.1016/s0301-7516(00)00045-4
Marhual NP, Pradhan N, Kar RN, Sukla LB, Mishra BK (2008) Differential bioleaching of copper by mesophilic and moderately thermophilic acidophilic consortium enriched from same copper mine water sample. Bioresour Technol 99(17):8331–8336
McGuire MM, Banfield JF, Hamers RJ (2001) Quantitative determination of elemental sulfur at the arsenopyrite surface after oxidation by ferric iron: mechanistic implications. Geochem Trans 2(4):25–29
McGuire MM, Hamers RJ (2000) Extraction and quantitative analysis of elemental sulfur from sulfide mineral surfaces by high-performance liquid chromatography. Environ Sci Technol 34(21):4651–4655
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59(3):695–700
Navarro CA, Orellana LH, Mauriaca C, Jerez CA (2009) Transcriptional and functional studies of Acidithiobacillus ferrooxidans genes related to survival in the presence of copper. Appl Environ Microbiol 75(19):6102–6109. doi:10.1128/aem.00308-09
Pogliani C, Curutchet G, Donati E, Tedesco PH (1990) A need for direct contact with particle surfaces in the bacterial oxidation of covellite in the absence of a chemical lixiviant. Biotechnol Lett 12(7):515–518. doi:10.1007/bf01086345
Porro S, Ramírez S, Reche C, Curutchet G, Alonso-Romanowski S, Donati E (1997) Bacterial attachment: its role in bioleaching processes. Process Biochem 32(7):573–578. doi:10.1016/s0032-9592(97)00018-6
Pradhan N, Nathsarma KC, Srinivasa Rao K, Sukla LB, Mishra BK (2008) Heap bioleaching of chalcopyrite: a review. Miner Eng 21(5):355–365. doi:10.1016/j.mineng.2007.10.018
Ramirez P, Toledo H, Guiliani N, Jerez CA (2002) An exported rhodanese-like protein is induced during growth of Acidithiobacillus ferrooxidans in metal sulfides and different sulfur compounds. Appl Environ Microbiol 68(4):1837–1845. doi:10.1128/aem.68.4.1837-1845.2002
Rodríguez Y, Ballester A, Blázquez ML, González F, Muñoz JA (2003) New information on the chalcopyrite bioleaching mechanism at low and high temperature. Hydrometallurgy 71(1–2):47–56
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl Microbiol Biotechnol 63(3):239–248. doi:10.1007/s00253-003-1448-7
Rohwerder T, Sand W (2003) The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology 149(7):1699–1710. doi:10.1099/mic.0.26212-0
Sampson MI, Blake RC (1999) The cell attachment and oxygen consumption of two strains of Thiobacillus ferrooxidans. Miner Eng 12(6):671–686. doi:10.1016/s0892-6875(99)00036-9
Sand W, Gehrke T (2006) Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res Microbiol 157(1):49–56. doi:10.1016/j.resmic.2005.07.012
Sand W, Gehrke T, Jozsa P-G, Schippers A (2001) (Bio)chemistry of bacterial leaching–direct vs. indirect bioleaching. Hydrometallurgy 59(2–3):159–175
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65(1):319–321
Schutz M, Maldener I, Griesbeck C, Hauska G (1999) Sulfide-quinone reductase from Rhodobacter capsulatus: requirement for growth, periplasmic localization, and extension of gene sequence analysis. J Bacteriol 181(20):6516–6523
Shrihari, Kumar R, Gandhi KS, Natarajan KA (1991) Role of cell attachment in leaching of chalcopyrite mineral by Thiobacillus ferrooxidans. Appl Microbiol Biotechnol 36(2):278–282
Shrihari, Modak JM, Kumar R, Gandhi KS (1995) Dissolution of particles of pyrite mineral by direct attachment of Thiobacillus ferrooxidans. Hydrometallurgy 38(2):175–187. doi:10.1016/0304-386x(94)00053-6
Silverman MP (1967) Mechanism of bacterial pyrite oxidation. J Bacteriol 94(4):1046–1051
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans II. J Bacteriol 78(3):326–331
Thurston RS, Mandernack KW, Shanks WC III (2010) Laboratory chalcopyrite oxidation by Acidithiobacillus ferrooxidans: oxygen and sulfur isotope fractionation. Chem Geol 269(3–4):252–261. doi:10.1016/j.chemgeo.2009.10.001
Vera M, Pagliai F, Guiliani N, Jerez CA (2008) The chemolithoautotroph Acidithiobacillus ferrooxidans can survive under phosphate-limiting conditions by expressing a C-P lyase operon that allows it to grow on phosphonates. Appl Environ Microbiol 74(6):1829–1835. doi:10.1128/aem.02101-07
Xia L, Liu X, Zeng J, Yin C, Gao J, Liu J, Qiu G (2008) Mechanism of enhanced bioleaching efficiency of Acidithiobacillus ferrooxidans after adaptation with chalcopyrite. Hydrometallurgy 92(3–4):95–101. doi:10.1016/j.hydromet.2008.01.002
Yarzábal A, Appia-Ayme C, Ratouchniak J, Bonnefoy V (2004) Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin. Microbiology 150(7):2113–2123. doi:10.1099/mic.0.26966-0
Yu R-l, Ou Y, Tan JX, Wu FD, Sun J, Miao L, Zhong D-l (2011) Effect of EPS on adhesion of Acidithiobacillus ferrooxidans on chalcopyrite and pyrite mineral surfaces. Trans Nonferr Metal Soc 21(2):407–412. doi:10.1016/s1003-6326(11)60729-2
Zeng W, Tan S, Chen M, Qiu G (2011) Detection and analysis of attached microorganisms on the mineral surface during bioleaching of pure chalcopyrite with moderate thermophiles. Hydrometallurgy 106(1–2):46–50. doi:10.1016/j.hydromet.2010.11.014
Zhu W, Xia J-l, Yang Y, Nie Z-Y, Zheng L, Ma C-Y, Zhang R-Y, Peng A-A, Tang L, Qiu G-Z (2011) Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite. Bioresour Technol 102(4):3877–3882. doi:10.1016/j.biortech.2010.11.090
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.30700008, 51174239) and the National Basic Research Program of China (No.2010CB630905).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Jiao, W., Li, Q. et al. Investigation of energy gene expressions and community structures of free and attached acidophilic bacteria in chalcopyrite bioleaching. J Ind Microbiol Biotechnol 39, 1833–1840 (2012). https://doi.org/10.1007/s10295-012-1190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1190-1