Abstract
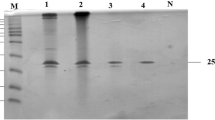
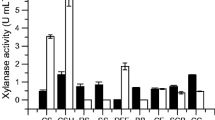
A xylanase-coding gene (xynAHJ3, 1,104 bp) was cloned from Lechevalieria sp. HJ3 harbored in a saline soil sampled from Heijing town, aka the “town of salt”, on the famous “Silk Route of the South”. The gene encodes a 367-residue polypeptide (XynAHJ3) with the highest identity of 74.0 % with the endoxylanase from Streptomyces thermocarboxydus HY-15. The coding sequence of the mature protein (without the predicted signal peptide from M1 to S22) of xynAHJ3 was expressed in Escherichia coli BL21 (DE3). The activity of the purified recombinant XynAHJ3 (rXynAHJ3) was apparently optimal at 70 °C and pH 6.0, retained greater than 55 % xylanase activity at a concentration of 0.2–2.0 M Na+ and 26 % at 4.0 M Na+ (pH 7.5 20 °C), and showed 110.2 and 44.2 % xylanase activities in the presence of 100 mM SDS (pH 6.0 37 °C) and 10 % ethanol (pH 5.0 37 °C), respectively. rXynAHJ3 activity was stable at 50 °C and pH 4.0–11.0 for more than 60 min, in trypsin or proteinase K at 20 °C for 24 h (pH 7.5), in 10 % ethanol (v/v) (pH 5.0) at 30 or 37 °C for 72 h, in 80 % ethanol (v/v) for 1 h, and in 0.6 or 3 M NaCl (20 °C, pH 7.5) for 72 h. Compared with the majority of xylanases with tolerance to ethanol, salt, SDS, or protease (K m values of 1.42–15.1 mg ml−1), rXynAHJ3 showed a low K m value (0.8 mg ml−1) and showed only limited amino acid sequence identity with those other xylanases (less than 47 %).






Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. doi:10.1006/jmbi.1990.9999
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56(3):326–338. doi:10.1007/s002530100704
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340(4):783–795. doi:10.1016/j.jmb.2004.05.028
Blanco J, Coque JJR, Velasco J, Martin JF (1997) Cloning, expression in Streptomyces lividans and biochemical characterization of a thermostable endo-β-1,4-xylanase of Thermomonospora alba ULJB1 with cellulose-binding ability. Appl Microbiol Biotechnol 48(2):208–217
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29(1):3–23. doi:10.1016/j.femsre.2004.06.005
Dhiman SS, Sharma J, Battan B (2008) Pretreatment processing of fabrics by alkalothermophilic xylanase from Bacillus stearothermophilus SDX. Enzyme Microb Technol 43(3):262–269. doi:10.1016/j.enzmictec.2008.03.016
Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer ELL, Bateman A (2008) The Pfam protein families database. Nucleic Acids Res 36:D281–D288. doi:10.1093/nar/gkm960
Guo B, Chen XL, Sun CY, Zhou BC, Zhang YZ (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl Microbiol Biotechnol 84(6):1107–1115. doi:10.1007/s00253-009-2056-y
Hung KS, Liu SM, Fang TY, Tzou WS, Lin FP, Sun KH, Tang SJ (2011) Characterization of a salt-tolerant xylanase from Thermoanaerobacterium saccharolyticum NTOU1. Biotechnol Lett 33(7):1441–1447. doi:10.1007/s10529-011-0579-7
Hung KS, Liu SM, Tzou WS, Lin FP, Pan CL, Fang TY, Sun KH, Tang SJ (2011) Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process Biochem 46(6):1257–1263. doi:10.1016/j.procbio.2011.02.009
Jaswal SS, Sohl JL, Davis JH, Agard DA (2002) Energetic landscape of α-lytic protease optimizes longevity through kinetic stability. Nature 415(6869):343–346. doi:10.1038/415343a
Kim DY, Han MK, Oh HW, Park DS, Kim SJ, Lee SG, Shin DH, Son KH, Bae KS, Park HY (2010) Catalytic properties of a GH10 endo-β-1,4-xylanase from Streptomyces thermocarboxydus HY-15 isolated from the gut of Eisenia fetida. J Mol Catal B Enzym 62(1):32–39. doi:10.1016/j.molcatb.2009.08.015
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Li CJ, Hong YZ, Shao ZZ, Lin L, Huang XL, Liu PF, Wu GB, Meng X, Liu ZD (2009) Novel alkali-stable, cellulase-free xylanase from deep-sea Kocuria sp. Mn22. J Microbiol Biotechnol 19(9):873–880. doi:10.4014/jmb.0812.689
Li N, Meng K, Wang YR, Shi PJ, Luo HY, Bai YG, Yang PL, Yao B (2008) Cloning, expression, and characterization of a new xylanase with broad temperature adaptability from Streptomyces sp. S9. Appl Microbiol Biotechnol 80 (2):231–240. doi:10.1007/s00253-008-1533-z
Li N, Yang PL, Wang Y, Luo HY, Meng K, Wu NF, Fan YL, Yao B (2008) Cloning, expression, and characterization of protease-resistant xylanase from Streptomyces fradiae var. k11. J Microbiol Biotechnol 18(3):410–416
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666. doi:10.1021/ja01318a036
Luo HY, Wang Y, Li J, Wang H, Yang J, Yang YH, Huang HQ, Fan YL, Yao B (2009) Cloning, expression and characterization of a novel acidic xylanase, XYL11B, from the acidophilic fungus Bispora sp. MEY-1. Enzyme Microb Technol 45(2):126–133. doi:10.1016/j.enzmictec.2009.05.002
Manning M, Colon W (2004) Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward β-sheet structure. Biochemistry 43(35):11248–11254. doi:10.1021/bi0491898
Menon G, Mody K, Keshri J, Jha B (2010) Isolation, purification, and characterization of haloalkaline xylanase from a marine Bacillus pumilus strain, GESF-1. Biotechnol Bioprocess Eng 15(6):998–1005. doi:10.1007/s12257-010-0116-x
Menon V, Prakash G, Prabhune A, Rao M (2010) Biocatalytic approach for the utilization of hemicellulose for ethanol production from agricultural residue using thermostable xylanase and thermotolerant yeast. Bioresour Technol 101(14):5366–5373. doi:10.1016/j.biortech.2010.01.150
Nassar N, Ortiz R (2010) Breeding cassava to feed the poor. Sci Am 302(5):78–84. doi:10.1038/scientificamerican0510-78
Ogino H, Ishikawa H (2001) Enzymes which are stable in the presence of organic solvents. J Biosci Bioeng 91(2):109–116. doi:10.1007/BF01022602
Oh HW, Heo SY, Kim DY, Park DS, Bae KS, Park HY (2008) Biochemical characterization and sequence analysis of a xylanase produced by an exo-symbiotic bacterium of Gryllotalpa orientalis, Cellulosimicrobium sp. HY-12. Antonie Leeuwenhoek 93(4):437–442. doi:10.1007/s10482-007-9210-2
Okoro CK, Bull AT, Mutreja A, Rong XY, Huang Y, Goodfellow M (2010) Lechevalieria atacamensis sp. nov., Lechevalieria deserti sp. nov. and Lechevalieria roselyniae sp. nov., isolated from hyperarid soils. Int J Syst Evol Microbiol 60(2):296–300. doi:10.1099/ijs.0.009985-0
Qiu Z, Shi P, Luo H, Bai Y, Yuan T, Yang P, Liu S, Yao B (2010) A xylanase with broad pH and temperature adaptability from Streptomyces megasporus DSM 41476, and its potential application in brewing industry. Enzyme Microb Technol 46(6):506–512. doi:10.1016/j.enzmictec.2010.02.003
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33:W116–W120. doi:10.1093/nar/gki442
Sato Y, Fukuda H, Zhou Y, Mikami S (2010) Contribution of ethanol-tolerant xylanase G2 from Aspergillus oryzae on Japanese sake brewing. J Biosci Bioeng 110(6):679–683. doi:10.1016/j.jbiosc.2010.07.015
Sinma K, Khucharoenphaisan K, Kitpreechavanich V, Tokuyama S (2011) Purification and characterization of a thermostable xylanase from Saccharopolyspora pathumthaniensis S582 isolated from the gut of a termite. Biosci Biotechnol Biochem 75(10):1957–1963. doi:10.1271/bbb.110353
Sornyotha S, Kyu KL, Ratanakhanokchai K (2010) An efficient treatment for detoxification process of cassava starch by plant cell wall-degrading enzymes. J Biosci Bioeng 109(1):9–14. doi:10.1016/j.jbiosc.2009.06.021
Tadeo X, Lopez-Mendez B, Trigueros T, Lain A, Castano D, Millet O (2009) Structural basis for the amino acid composition of proteins from halophilic Archea. PLoS Biol 7(12):e1000257. doi:10.1371/journal.pbio.1000257
Wang W, Zhang ZD, Tang QY, Mao J, Wei D, Huang Y, Liu ZH, Shi YH, Goodfellow M (2007) Lechevalieria xinjiangensis sp. nov., a novel actinomycete isolated from radiation-polluted soil in China. Int J Syst Evol Microbiol 57(12):2819–2822. doi:10.1099/ijs.0.65134-0
Wi SG, Kim HJ, Mahadevan SA, Yang DJ, Bae HJ (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100(24):6658–6660. doi:10.1016/j.biortech.2009.07.017
Wu J, Guan T, Jiang H, Zhi X, Tang S, Dong H, Zhang L, Li W (2009) Diversity of Actinobacterial community in saline sediments from Yunnan and Xinjiang, China. Extremophiles 13(4):623–632. doi:10.1007/s00792-009-0245-3
Zhou JP, Huang HQ, Meng K, Shi PJ, Wang YR, Luo HY, Yang PL, Bai YG, Yao B (2010) Cloning of a new xylanase gene from Streptomyces sp. TN119 using a modified thermal asymmetric interlaced-PCR specific for GC-rich genes and biochemical characterization. Appl Biochem Biotechnol 160(5):1277–1292. doi:10.1007/s12010-009-8642-8
Zhou JP, Huang HQ, Meng K, Shi PJ, Wang YR, Luo HY, Yang PL, Bai YG, Zhou ZG, Yao B (2009) Molecular and biochemical characterization of a novel xylanase from the symbiotic Sphingobacterium sp. TN19. Appl Microbiol Biotechnol 85 (2):323–333. doi:10.1007/s00253-009-2081-x
Zhou JP, Shi PJ, Zhang R, Huang HQ, Meng K, Yang PL, Yao B (2011) Symbiotic Streptomyces sp. TN119 GH 11 xylanase: a new pH-stable, protease- and SDS-resistant xylanase. J Ind Microbiol Biotechnol 38(4):523–530. doi:10.1007/s10295-010-0795-5
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program; No. 2008AA02Z202), National Natural Science Foundation of China (No. 31160229), Applied and Basic Research Foundation of Yunnan Province (No. 2011FB048), and Foundation of Yunnan Normal University (No. 11ZQ07).
Author information
Authors and Affiliations
Corresponding author
Additional information
Junpei Zhou and Yajie Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, J., Gao, Y., Dong, Y. et al. A novel xylanase with tolerance to ethanol, salt, protease, SDS, heat, and alkali from actinomycete Lechevalieria sp. HJ3. J Ind Microbiol Biotechnol 39, 965–975 (2012). https://doi.org/10.1007/s10295-012-1113-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1113-1