Abstract
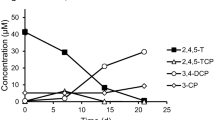
Two rapidly growing propionibacteria that could reductively dechlorinate tetrachloroethylene (PCE) and cis-1,2-dichloroethylene (cis-DCE) to ethylene were isolated from environmental sediments. Metabolic characterization and partial sequence analysis of their 16S rRNA genes showed that the new isolates, designated as strains Propionibacterium sp. HK-1 and Propionibacterium sp. HK-3, did not match any known PCE- or cis-DCE-degrading bacteria. Both strains dechlorinated relatively high concentrations of PCE (0.3 mM) and cis-DCE (0.52 mM) under anaerobic conditions without accumulating toxic intermediates during incubation. Cell-free extracts of both strains catalyzed PCE and cis-DCE dechlorination; degradation was accelerated by the addition of various electron donors. PCE dehalogenase from strain HK-1 was mediated by a corrinoid protein, since the dehalogenase was inactivated by propyl iodide only after reduction by titanium citrate. The amounts of chloride ions (0.094 and 0.103 mM) released after PCE (0.026 mM) and cis-DCE (0.05 mM) dehalogenation using the cell-free enzyme extracts of both strains, HK-1 and HK-3, were stoichiometrically similar (91 and 100%), indicating that PCE and cis-DCE were fully dechlorinated. Radiotracer studies with [1,2-14C] PCE and [1,2-14C] cis-DCE indicated that ethylene was the terminal product; partial conversion to ethylene was observed. Various chlorinated aliphatic compounds (PCE, trichloroethylene, cis-DCE, trans-1,2-dichloroethylene, 1,1-dichloroethylene, 1,1-dichloroethane, 1,2-dichloroethane, 1,2-dichloropropane, 1,1,2-trichloroethane, and vinyl chloride) were degraded by cell-free extracts of strain HK-1.





Similar content being viewed by others
References
Adrian L, Szewzyk U, Wecke J, Gorisch H (2000) Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580–583
Bouwer EJ, McCarty PL (1983) Transformation of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol 45:1286–1294
Bradley PM, Chapelle FH (1997) Kinetics of DCE and VC mineralization under methanogenic and Fe(III)-reducing conditions. Environ Sci Technol 31:2692–2696
Bradley PM, Chapelle FH (1998) Microbial mineralization of VC and DCE under different terminal electron accepting conditions. Anaerobe 4:81–87
Bradley PM, Chapelle FH, Lovley DR (1998) Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloroethene. Appl Environ Microbiol 64:3102–3105
Brot N, Weissbach H (1965) Enzymatic synthesis of methionine; chemical alkylation of the enzyme-bound cobamide. J Biol Chem 240:3064–3070
Chang YC, Hatsu M, Jung K, Yoo YS, Takamizawa K (2000) Isolation and characterization of a tetrachloroethylene dechlorinating bacterium, Clostridium bifermentans DPH-1. J Biosci Bioeng 89:489–491
Chang YC, Okeke BC, Hatsu M, Takamizawa K (2001) In vitro dehalogenation of tetrachloroethylene (PCE) by cell-free extracts of Clostridium bifermentans DPH-1. Bioresour Technol 78:141–147
Damborsky J (1999) Tetrachloroethene-dehalogenating bacteria. Folia Microbiol 44:247–262
Dennie D, Gladu I, Lepine F, Villemur R, Bisaillon JG, Beaudet R (1998) Spectrum of the reductive dehalogenation activity of Desulfitobacterium frappieri PCP-1. Appl Environ Microbiol 64:4603–4606
Ernest MU, Gabarrell X, Sarrà M, Caminal G, Vicent T, Reddy CA (2006) Novel aerobic perchloroethylene degradation by the white-rot fungus Trametes versicolor. Environ Sci Technol 40:7796–7802
Fetzner S (1998) Bacterial dehalogenation. Appl Microbiol Biotechnol 50:633–657
Fletcher KE, Ritalahti KM, Pennell KD, Takamizawa K, Löffler FE (2008) Resolution of culture Clostridium bifermentans DPH-1 into two populations, a Clostridium sp. and tetrachloroethene-dechlorinating Desulfitobacterium hafniense strain JH1. Appl Environ Microbiol 74:6141–6143
Freedman DL, Gossett JM (1989) Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol 55:2144–2151
Friedrich W (1975) Vitamin B12 und verwandte corrinoid. Thieme, Stuttgart
Gerritse J, Renard V, Pedro Gomes TM, Lawson PA, Collins MD, Gottschal JC (1996) Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethylene or ortho-chlorinated phenols. Arch Microbiol 165:132–140
Gerritse J, Drzyzga O, Kloetstra G, Keijmel M, Wiersum LP, Hutson R, Collins MD, Gottschal JC (1999) Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol 65:5212–5221
Gossett JM (1987) Measurement of Henry’s law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol 21:202–208
Gossett JM (2002) Fishing for microbes. Science 298:974–975
Hata J, Miyata N, Kim ES, Takamizawa K, Iwahori K (2004) Anaerobic degradation of cis-1,2-dichloroethylene and vinyl chloride by Clostridium sp. Strain DC1 isolated from landfill leachate sediment. J Biosci Bioeng 97:196–201
He J, Ritalahti KM, Aiello MR, Löffler FE (2003) Complete detoxification of vinyl chloride by an anaerobic enrichment culture and identification of the reductively dechlorinating population as a Dehalococcoides species. Appl Environ Microbiol 69:996–1003
He J, Ritalahti KM, Yang KL, Koenigsberg SS, Löffler FE (2003) Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62–65
He J, Sung Y, Krajmalnik-Brown R, Ritalahti KM, Löffler FE (2005) Isolation and characterization of Dehalococcoides sp. strain FL2, a trichloroethene (TCE)- and 1,2-dichloroethene-respiring anaerobe. Environ Microbiol 7:1442–1450
Hendrickson ER, Payne JA, Young RM, Starr MG, Perry MP, Fahnestock S, Ellis DE, Ebersole RC (2002) Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout north America and Europe. Appl Environ Microbiol 68:485–495
Hippe H, Vainshtein M, Gogotova GI, Stackebrandt E (2003) Reclassification of Desulfobacterium macestii as Desulfomicrobium macestii comb. Nov. Int J Syst Evol Microbiol 53:1127–1130
Holliger C, Schraa G, Stams AJM, Zehnder AJB (1993) A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl Environ Microbiol 59:2991–2997
Holliger C, Hahn D, Harmsen H, Ludwig W, Schumacher W, Tindall B, Vazquez F, Weiss N, Zehnder AJB (1998) Dehalobacter restrictus gen. nov. and sp. nov., a stirictly anaerobic bacterium that reductively dechlorinates tetrachloroethene and trichloroethene in an anaerobic respiration. Arch Microbiol 169:313–321
Ito Y, Suzuki K, Ando T, Nashimoto K, Niwa K, Shimada K (2007) Technology for detection of degradation microorganisms and bioremediation. Matsushita Techn J 53:16–21
Jablonski PE, Ferry JG (1992) Reductive dechlorination of trichloroethylene by the CO-reduced CO dehydrogenase enzyme complex from Methanosarcina thermophila. FEMS Microbiol Lett 96:55–60
Kim ES, Nomura I, Hasegawa Y, Takamizawa K (2006) Characterization of a newly isolated cis-1,2-dichloroethylene and aliphatic compound-degrading bacterium, Clostridium sp. strain KYT-1. Biotechnol Bioprocess Eng 11:553–556
Krumholz LR (1997) Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int J Syst Bacteriol 47:1262–1263
Leadbetter JR (2005) Environmental microbiology. In: Löffler FE, Sanford RA, Ritalahti KM (eds) Methods in enzymology, vol 397. Elsevier, Amsterdam, pp 77–111
Löffler FE, Tiedje JM, Sanford RA (1999) Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl Environ Microbiol 65:4049–4056
Löffler FE, Sanford RA, Tiedje JM (1996) Initial characterization of a reductive dehalogenase from Desulfitobacterium chlororespirans Co23. Appl Environ Microbiol 62:3809–3813
Luijten MLGC, Weert JD, Smidt H, Boschker HTS, Vos WMD, Schraa G, Stams AJM (2003) Description of Sulfurospirillum halorespirans sp. nov., an anaerobic, tetrachloroethene-respiring bacterium, and transfer of Dehalospirillum multivorans to the genus Sulfurospirillum as Sulfurospirillum multivorans comb. nov. Int J Syst Evol Microbiol 53:787–793
Maillard J, Schumacher W, Vazquez F, Regeard C, Hagen WR, Holliger C (2003) Characterization of the corrinoid iron-sulfur protein tetrachloroethene reductive dehalogenase of Dehalobacter restrictus. Appl Environ Microbiol 69:4628–4638
Maymó-Gatell X, Tandoi V, Gossett JM, Zinder SH (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethane. Science 276:1568–1571
Miller E, Wohlfarth G, Diekert G (1996) Studies on tetrachloroethene respiration in Dehalospirillum multivorans. Arch Microbiol 166:379–387
Miller E, Wohlfarth G, Diekert G (1998) Comparative studies on tetrachloroethene reductive dechlorination mediated by Desulfitobacterium sp. strain PCE-S. Arch Microbiol 168:513–519
Mohn WW, Kennedy KJ (1992) Reductive dehalogenation of chlorophenols by Desulfomonile tiedjei DCB-1. Appl Environ Microbiol 58:1367–1370
Neumann A, Wohlfarth G, Diekert G (1995) Properties of tetrachloroethene dehalogenase of Dehalospirillum multivorans. Arch Microbiol 163:276–281
Phelps TJ, Malachowsky K, Schram RM, White DC (1991) Aerobic mineralization of vinyl chloride by a bacterium of the order Actinomycetales. Appl Environ Microbiol 57:1252–1254
Ryoo D, Shim H, Canada K, Barbieri P, Wood TK (2000) Aerobic degradation of tetrachloroethylene by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Nat Biotechnol 18:775–778
Sanford RA, Cole JR, Löffler FE, Tiedje JM (1996) Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol 62:3800–3808
Scholz-Muramatsu H, Neumann A, Meßmer M, Moore E, Diekert G (1995) Isolation and characterization of Dehalospirillum multivorans gen. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol 163:48–56
Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe BA, Ward NL, Nelson WC, Deboy RT, Khouri HM, Kolonay JF, Dodson RJ, Daugherty SC, Brinkac LM, Sullivan SA, Madupu R, Nelson KE, Kang KH, Impraim M, Tran K, Robinson JM, Forberger HA, Fraser CM, Zinder SH, Heidelberg JF (2005) Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science 307:105–108
Sharma PK, McCarty PL (1996) Isolation and characterization of a facultatively aerobic bacterium that reductively dehalogenates tetrachloroethene to cis-1,2-dichloroethene. Appl Environ Microbiol 62:761–765
Sung Y, Ritalahti KM, Sanford RA, Urbance JW, Flynn SJ, Tiedje JM, Löffler FE (2003) Characterization of two tetrachloroethene (PCE)-reducing, acetate-oxidizing anaerobic bacteria, and their description as Desulfuromonas michiganensis sp. nov. Appl Environ Microbiol 69:2964–2974
Sung Y, Fletcher KE, Ritalahti KM, Apkarian RP, Ramos-Hernández N, Sanford RA, Mesbah NM, Löffler FE (2006) Geobacter lovleyi sp. nov. strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium. Appl Environ Microbiol 72:2775–2782
Sung Y, Ritalahti KM, Apkarian RP, Löffler FE (2006) Quantitative PCR confirms purity of strain GT, a novel trichloroethene-to-ethene-respiring Dehalococcoides isolate. Appl Environ Microbiol 72:1980–1987
Suyama A, Iwakiri R, Kai K, Tokunaga T, Sera N, Furukawa K (2001) Isolation and characterization of Desulfitobacterium sp. strain Y51 capable of efficient dechlorination of tetrachloroethene and polychloroethanes. Biosci Biotechnol Biochem 65:1474–1481
Terzenbach DP, Blaut M (1994) Transformation of tetrachloroethylene to trichloroethylene by homoacetogenic bacteria. FEMS Microbiol Lett 123:213–218
Thorenoor N, Kim YH, Lee CJ, Yu MH, Engesser KH (2009) A previously uncultured, paper mill Propionibacterium is able to degrade O-aryl alkyl ethers and various aromatic hydrocarbons. Chemosphere 75:1287–1293
Tsukagoshi N, Ezaki S, Uenaka T, Suzuki N, Kurane R (2006) Isolation and transcriptional analysis of novel tetrachloroethene reductive dehalogenase gene from Desulfitobacterium sp. strain KBC1. Appl Microbiol Biotechnol 69:543–553
Utkin IY, Woese C, Wiegel J (1994) Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol 44:612–619
Yoshida N, Asahi K, Sakakibara Y, Miyake K, Katayama A (2007) Isolation and quantitative detection of tetrachloroethene (PCE)-dechlorinating bacteria in unsaturated subsurface soils contaminated with chloroethenes. J Biosci Bioeng 104:91–97
Acknowledgments
This research was supported by a grant [Research for Promoting Technological Seeds (A), no. 01-044] from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chang, YC., Ikeutsu, K., Toyama, T. et al. Isolation and characterization of tetrachloroethylene- and cis-1,2-dichloroethylene-dechlorinating propionibacteria. J Ind Microbiol Biotechnol 38, 1667–1677 (2011). https://doi.org/10.1007/s10295-011-0956-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0956-1