Abstract
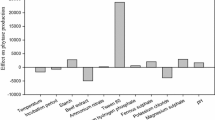
Phytase production by Aspergillus niger NCIM 563 was optimized by using wheat bran in solid state fermentation (SSF). An integrated statistical optimization approach involving the combination of Placket–Burman design (PBD) and Box–Behnken design (BBD) was employed. PBD was used to evaluate the effect of 11 variables related to phytase production, and five statistically significant variables, namely, glucose, dextrin, NaNO3, distilled water, and MgSO4·7H2O, were selected for further optimization studies. The levels of five variables for maximum phytase production were determined by a BBD. Phytase production improved from 50 IU/g dry moldy bran (DMB) to 154 IU/g DMB indicating 3.08-fold increase after optimization. A simultaneous reduction in fermentation time from 7 to 4 days shows a high productivity of 38,500 IU/kg/day. Scaling up the process in trays gave reproducible phytase production overcoming industrial constraints of practicability and economics. The culture extract also had 133.2, 41.58, and 310.34 IU/g DMB of xylanase, cellulase, and amylase activities, respectively. The partially purified phytase was optimally active at 55°C and pH 6.0. The enzyme retained ca. 75% activity over a wide pH range 2.0–9.5. It also released more inorganic phosphorus from soybean meal in a broad pH range from 2.5 to 6.5 under emulated gastric conditions. Molecular weight of phytase on Sephacryl S-200 was approximately 87 kDa. The K m and V max observed were 0.156 mM and 220 μm/min/mg. The SSF phytase from A. niger NCIM 563 offers an economical production capability and its wide pH stability shows its suitability for use in poultry feed.




Similar content being viewed by others
References
Andrews P (1964) Estimation of molecular weight of proteins by Sephadex gel filtration. Biochem J 92:222–223
Bogar B, Szakacs G, Tengerdy RP, Linden JC, Pandey A (2003) Optimization of phytase production by solid substrate fermentation. J Ind Microbiol Biotech 30:183–189
Bogar B, Szakacs G, Pandey A, Abdulhameed S, Linden J, Tengerdy R (2003) Production of phytase by Mucor racemosus in solid state fermentation. Biotechnol Prog 19:312–319
Box GEP, Hunter JS (1957) Multifactor experimental design for exploring response surfaces. Ann Math Stat 28:95–241
Chadha BS, Gulati H, Mandeep M, Saini SH, Singh N (2004) Phytase production by the thermophilic fungus Rhizomucor pusillus. World J Microbiol Biotechnol 20:105–109
Davis BJ (1964) Disc electrophoresis II. Method and application to human serum proteins. Ann N Y Acad Sci 121:404–427
Deutscher MP (1990) Guide to protein purification, methods enzymology, vol 182. Academic, Toronto, p 430
Durand D, Broise D, Blachere H (1988) Laboratory scale bioreactor for solid state process. J Biotechnol 8:59–66
Garrett JB, Kretz KA, O’Donoghue E, Kerovuo J, Kim W, Barton NR, Hazlewood GP, Short JM, Robertson DE, Gray KA (2004) Enhancing the thermal tolerance and gastric performance of a microbial phytase for use as a phosphate-mobilizing monogastric-feed supplement. Appl Environ Microbiol 70:3041–3046
Gilati HK, Chadha BS, Saini HS (2007) Production of feed enzymes (phytase and plant cell wall hydrolyzing enzymes) by Mucor indicus MTCC 6333: purification and characterization of phytase. Folia Microbiol 52:491–497
Gokhale DV, Puntambekar US, Deobagkar DN, Peberdy JF (1988) Production of cellulolytic enzymes by mutants of Aspergillus niger NCIM 1207. Enzy Microbiol Technol 10:442–445
Gunashree BS, Venkateswaran G (2008) Effect of different cultural conditions for phytase production by Aspergillus niger CFR 335 in submerged and solid-state fermentations. J Ind Microbiol Biotechnol 35:1587–1596
Huoqing H, Huiying L, Wang Y, Fu D, Shao N, Wang G, Yang P, Yao B (2008) A novel phytase from Yersinia rohdei with high phytate hydrolysis activity under low pH and strong pepsin conditions. Appl Microbiol Biotechnol 80:417–426
Konietzny U, Greiner R (2002) Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int J Food Sci Technol 37:791–812
Krishna C, Nokes SE (2001) Predicting vegetative inoculum performance to maximize phytase production in solid-state fermentation using response surface methodology. J Ind Microbiol Biotechnol 26:161–170
Lineweaver H, Burk D (1934) Determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lei X, Stahl CH (2001) Biotechnological development of effective phytases for mineral nutrition and environmental protection. Appl Microbiol Biotechnol 57:474–481
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mandviwala TN, Khire JM (2000) Production of high activity thermostable phytase from thermotolerant Aspergillus niger in solid-state fermentation. J Ind Micrbiol Biotechnol 24:237–243
Miller GL, Lorenz G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
McCleary BV, Sheehan H (1987) Measurement of cereal alpha amylase: a new assay procedure. J Cereal Sci 6:237–251
Ole K, Torben VB, Claus CF (2002) Industrial enzyme applications. Curr Opinion Biotechnol 13:345–351
Omogbenigun FO, Nyachoti CM, Slominski BA (2004) Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. J Anim Sci 82:1053–1061
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation. Proc Biochem 35:397–402
Pandey A, Szakacs G, Soccol CR, Rodriguez-Leon JA, Soccol VT (2001) Production, purification and properties of microbial phytases. Bioresour Technol 77:203–214
Plackett RL, Burman JP (1946) The design of optimum multifactor experiments. Biometrika 33:305–325
Prabhakar A, Krishnaiah K, Janaun J, Bono A (2005) An overview of engineering aspects of solid state fermentation. Malaysian J Microbiol 1:10–16
Raboy V (2001) Seeds for a better future: “low phytate” grains help to overcome malnutrition and pollution. Trend Plant Sci 6:458–462
Reissig JL, Stromiger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217:959–966
Singh B, Satyanarayan T (2006) A marked enhancement in phytase production by a thermophilic mould Sporotricum thermophile using statistical designs in a cost effective cane molasses medium. J Appl Microbiol 101:344–352
Soni SK, Khire JM (2007) Production and partial characterization of two types of phytase from Aspergillus niger NCIM 563 under SF conditions. World J Microbiol Biotechnol 23:1585–1593
Terebiznik MR, Pilosof AMR (1999) Biomass estimation in solid state fermentation by modeling dry matter weight loss. Biotech Tech 13:215–221
Vats P, Banerjee UC (2004) Production studies and catalytic properties of phytases (myoinositolhexakisphosphate phosphohydrolases): an overview. Enzy. Microbiol Technol 35:3–14
Vohra A, Satyanarayana T (2003) Phytases: microbial sources, production, purification and potential biotechnological applications. Crit Rev in Biotech 23:29–60
Wodzinski RJ, Ullah AHJ (1996) Phytase. Adv Appl Microbiol 42:263–302
Wu YB, Ravindran V, Thomas DG, Birtles MJ, Hendricks WH (2004) Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolizable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. Brit Poultry Sci 45:76–84
Acknowledgments
One of the authors, Ms Kavita Bhavsar, thanks Council of Scientific and Industrial Research, Government of India for the financial assistance. We also gratefully acknowledge support and facilities provided by the Center of Excellence in Scientific Computing, National Chemical Laboratory, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhavsar, K., Ravi Kumar, V. & Khire, J.M. High level phytase production by Aspergillus niger NCIM 563 in solid state culture: response surface optimization, up-scaling, and its partial characterization. J Ind Microbiol Biotechnol 38, 1407–1417 (2011). https://doi.org/10.1007/s10295-010-0926-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0926-z