Abstract
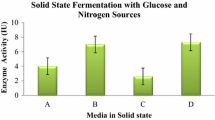
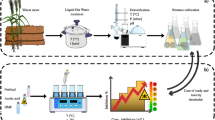
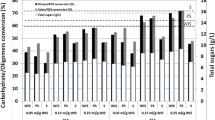
Pretreatment is a necessary step in the biomass-to-ethanol conversion process. The side stream of the pretreatment step is the liquid fraction, also referred to as the hydrolyzate, which arises after the separation of the pretreated solid and is composed of valuable carbohydrates along with compounds that are potentially toxic to microbes (mainly furfural, acetic acid, and formic acid). The aim of our study was to utilize the liquid fraction from steam-exploded wheat straw as a carbon source for cellulase production by Trichoderma reesei RUT C30. Results showed that without detoxification, the fungus failed to utilize any dilution of the hydrolyzate; however, after a two-step detoxification process, it was able to grow on a fourfold dilution of the treated liquid fraction. Supplementation of the fourfold-diluted, treated liquid fraction with washed pretreated wheat straw or ground wheat grain led to enhanced cellulase (filter paper) activity. Produced enzymes were tested in hydrolysis of washed pretreated wheat straw. Supplementation with ground wheat grain provided a more efficient enzyme mixture for the hydrolysis by means of the near-doubled β-glucosidase activity obtained.



Similar content being viewed by others
References
Almeida JRM, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349. doi:10.1002/jctb.1676
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. doi:10.1016/j.biortech.2009.11.093
Ballesteros I, Negro MJ, Oliva JM, Cabanas A, Manzanares P, Ballesteros M (2006) Ethanol production from steam-explosion pretreated wheat straw. Appl Biochem Biotechnol 129–132:496–508. doi:10.1385/ABAB:130:1:496
Benitez T, Limón C, Delgado-Jarana J, Rey M (1998) Glucanolytic and other enzymes and their genes. In: Harman GE, Kubicek CP (eds) Trichoderma and gliocladium, vol 2. Taylor & Francis, London, UK, pp 89–113
Berghem LE, Pettersson LG (1974) The mechanism of enzymatic cellulose degradation. Isolation and some properties of a β-glucosidase from Trichoderma viride. Eur J Biochem 46:295–305. doi:10.1111/j.1432-1033.1974.tb03621.x
Berlin A, Gilkes N, Kilburn D, Bura R, Markov A, Skomarovsky A, Okunev O, Gusakov A, Maximenko V, Gregg D, Sinitsyn A, Saddler J (2005) Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates—evidence for the role of accessory enzymes. Enzyme Microb Technol 37:175–184. doi:10.1016/j.enzmictec.2005.01.039
Bigelow M, Wyman C (2002) Cellulase production on bagasse pretreated with hot water. Appl Biochem Biotechnol 98–100:921–934. doi:10.1385/ABAB:98-100:1-9:921
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Breuil C, Chan M, Gilbert M, Saddler JN (1992) Influence of β-glucosidase on the filter paper activity and hydrolysis of lignocellulosic substrates. Bioresour Technol 39:139–142. doi:10.1016/0960-8524(92)90132-H
Chen S, Wayman M (1992) Novel inducers derived from starch for cellulase production by Trichoderma reesei. Process Biochem 27:327–334. doi:10.1016/0032-9592(92)87010-E
Fujimoto H, Nishida H, Ajisaka K (1988) Enzymatic syntheses of glucobioses by a condensation reaction with α-glucosidase, β-glucosidases and glucoamylase. Agric Biol Chem 52:1345–1351
Hayward T, Hamilton J, Templeton D, Jennings E, Ruth M, Tholudur A, McMillan J, Tucker M, Mohagheghi A (1999) Enzyme production, growth, and adaptation of T. reesei strains QM9414, L-27, RL-P37, and RUT C-30 to conditioned yellow poplar sawdust hydrolysate. Appl Biochem Biotechnol 77:293–309. doi:10.1385/ABAB:77:1-3:293
Hägglund E (1951) The wood components and their chemical properties. In: Hägglund E (ed) Chemistry of wood. Academic, New York, USA, pp 37–389
Horváth IS, Sjöde A, Alriksson B, Jönsson LJ, Nilvebrant N (2005) Critical conditions for improved fermentability during overliming of acid hydrolysates from spruce. Appl Biochem Biotechnol 121–124:1031–1044. doi:10.1007/978-1-59259-991-2_87
Ilmén M, Thrane C, Penttilä M (1996) The glucose repressor gene cre1 of Trichoderma: isolation and expression of a full-length and a truncated mutant form. Mol Gen Genet 251:451–460. doi:10.1007/BF02172374
Jorgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1:119–134. doi:10.1002/bbb.4
Juhász T, Szengyel Z, Szijártó N, Réczey K (2004) Effect of pH on cellulase production of Trichoderma reesei RUT C30. Appl Biochem Biotechnol 113:201–211. doi:10.1385/ABAB:113:1-3:201
Kaparaju P, Serrano M, Thomsen AB, Kongjan P, Angelidaki I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour Technol 100:2562–2568. doi:10.1016/j.biortech.2008.11.011
Kaparaju P, Serrano M, Angelidaki I (2009) Effect of reactor configuration on biogas production from wheat straw hydrolysate. Bioresour Technol 100:6317–6323. doi:10.1016/j.biortech.2009.06.101
Kovács K, Szakacs G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Bioresour Technol 100:1350–1357. doi:10.1016/j.biortech.2008.08.006
Kumar S, Singh SP, Mishra IM, Adhikari DK (2009) Recent advances in production of bioethanol from lignocellulosic biomass. Chem Eng Technol 32:517–526. doi:10.1002/ceat.200800442
Larsson S, Reimann A, Nilvebrant N, Jönsson L (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77:91–103. doi:10.1385/ABAB:77:1-3:91
Lo C, Zhang Q, Lee P, Ju L (2005) Cellulase production by Trichoderma reesei using sawdust hydrolysate. Appl Biochem Biotechnol 121–124:561–573. doi:10.1385/ABAB:122:1-3:0561
Marchessault RH, Malhotra SL, Jones AY, Perovic A (1983) The wood explosion process: characterization and uses of lignin/cellulose products. In: Wood and agriculture residues. Academic, New York, pp 401–413
Marquina D, Flores M (1997) Use of potato starch extraction wastes to grow cellulolytic fungi. Adv Food Sci 19:75–80
Messner R, Kubicek CP (1990) Evidence for a single, specific β-glucosidase in cell walls from Trichoderma reesei QM9414. Enzyme Microb Technol 12:685–690. doi:10.1016/0141-0229(90)90008-E
Mohagheghi A, Grohmann K, Wyman C (1988) Production of cellulase on mixtures of xylose and cellulose. Appl Biochem Biotechnol 17:263–277. doi:10.1007/BF02779162
Nogawa M, Goto M, Okada H, Morikawa Y (2001) l-Sorbose induces cellulase gene transcription in the cellulolytic fungus Trichoderma reesei. Curr Genet 38:329–334. doi:10.1007/s002940000165
Olsson L, Hahn-Hägerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol 18:312–331. doi:10.1016/0141-0229(95)00157-3
Palmqvist E, Hahn-Hägerdal B, Szengyel Z, Zacchi G, Réczey K (1997) Simultaneous detoxification and enzyme production of hemicellulose hydrolysates obtained after steam pretreatment. Enzyme Microb Technol 20:286–293. doi:10.1016/S0141-0229(96)00130-5
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24. doi:10.1016/S0960-8524(99)00160-1
Parajó JC, Domínguez H, Domínguez JM (1998) Biotechnological production of xylitol part. 3: Operation in culture media made from lignocellulose hydrolysates. Bioresour Technol 66:25–40. doi:10.1016/S0960-8524(98)00037-6
Réczey K, Brumbauer A, Bollók M, Szengyel Z, Zacchi G (1998) Use of hemicellulose hydrolysate for β-glucosidase fermentation. Appl Biochem Biotechnol 70–72:225–235. doi:10.1007/BF02920139
Seidl V, Gamauf C, Druzhinina IS, Seiboth B, Hartl L, Kubicek CP (2008) The Hypocrea jecorina (Trichoderma reesei) hypercellulolytic mutant RUT C30 lacks a 85 kb (29 gene-encoding) region of the wild-type genome. BMC Genomics 9:327. doi:10.1186/1471-2164-9-327
Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol 139:761–769
Szengyel Z, Zacchi G, Réczey K (1997) Cellulase production based on hemicellulose hydrolysate from steam-pretreated willow. Appl Biochem Biotechnol 63–65:351–362. doi:10.1007/BF02920437
Szengyel Z, Zacchi G (2000) Effect of acetic acid and furfural on cellulase production of Trichoderma reesei RUT C30. Appl Biochem Biotechnol 89:31–42. doi:10.1385/ABAB:89:1:31
Taj-Aldeen SJ (1993) Effect of starch on the induction of β-glucosidase in Trichoderma reesei. Mycol Res 97:318–320. doi:10.1016/S0953-7562(09)81128-3
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101:4744–4753. doi:10.1016/j.biortech.2009.11.080
van Maris AJA, Abbott DA, Bellissimi E, van den Brink J, Kuyper M, Luttik MAH, Wisselink HW, Scheffers WA, van Dijken JP, Pronk JT (2006) Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie van Leeuwenhoek 90:391–418. doi:10.1007/s10482-006-9085-7
Wang CH, Hseu TH, Huang CM (1988) Induction of cellulase by cello-oligosaccharides in Trichoderma koningii G-39. J Biotechnol 9:47–59. doi:10.1016/0168-1656(88)90014-4
Warzywoda M, Larbre E, Pourquié J (1992) Production and characterization of cellulolytic enzymes from Trichoderma reesei grown on various carbon sources. Bioresour Technol 39:125–130. doi:10.1016/0960-8524(92)90130-P
Wayman M, Chen S (1992) Cellulase production by Trichoderma reesei using whole wheat flour as a carbon source. Enzyme Microb Technol 14:825–831. doi:10.1016/0141-0229(92)90099-A
Xiong H, Turunen O, Pastinen O, Leisola M, von Weymarn N (2004) Improved xylanase production by Trichoderma reesei grown on l-arabinose and lactose or d-glucose mixtures. Appl Microbiol Biotechnol 64:353–358. doi:10.1007/s00253-003-1548-4
Xiong H, von Weymarn N, Turunen O, Leisola M, Pastinen O (2005) Xylanase production by Trichoderma reesei RUT C-30 grown on l-arabinose-rich plant hydrolysates. Bioresour Technol 96:753–759. doi:10.1016/j.biortech.2004.08.007
Acknowledgments
Hungarian National Research Fund (OTKA K72710) is gratefully acknowledged for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gyalai-Korpos, M., Mangel, R., Alvira, P. et al. Cellulase production using different streams of wheat grain- and wheat straw-based ethanol processes. J Ind Microbiol Biotechnol 38, 791–802 (2011). https://doi.org/10.1007/s10295-010-0811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0811-9