Abstract
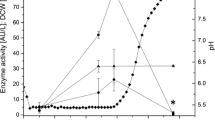
A gene encoding the thermostable α-amylase in Thermobifida fusca NTU22 was amplified by PCR, sequenced, and cloned into Yarrowia lipolytica P01g host strain using the vector pYLSC1 allowing constitutive expression and secretion of the protein. Recombinant expression resulted in high levels of extracellular amylase production, as high as 730 U/l in the Hinton flask culture broth. It is higher than that observed in P. pastoris expression system and E. coli expression system. The purified amylase showed a single band at about 65 kDa by SDS-polyacrylamide gel electrophoresis and this agrees with the predicted size based on the nucleotide sequence. About 70% of the original activity remained after heat treatment at 60°C for 3 h. The optimal pH and temperature of the purified amylase were 7.0 and 60°C, respectively. The purified amylase exhibited a high level of activity with raw sago starch. After 72-h treatment, the DP w of raw sago starch obviously decreased from 830,945 to 237,092. The boiling stable resistant starch content of the sago starch increased from 8.3 to 18.1%. The starch recovery rate was 71%.






Similar content being viewed by others
References
Henrissat B (1991) A classification of glycohydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Nguyen QD, Rezessy-Szabo JM, Claeyssens M, Stals I, Hoschke A (2002) Purification and characterization of amylolytic enzymes from thermophilic fungus Thermomyces lanuginosus strain ATCC 34626. Enzyme Microb Technol 31:345–352
Liu WH, Yang CH (2002) The isolation and identification of a lignocellulolytic and thermophilic actinomycete. Food Sci Agric Chem 4:89–94
Yang CH, Cheng KC, Liu WH (2003) Optimization of medium composition for production of extracellular amylase by Thermobifida fusca using a response surface methodology. Food Sci Agric Chem 5:35–40
Yang CH, Liu WH (2004) Purification and properties of a maltotriose-producing α-amylase from Thermobifida fusca. Enzyme Microb Technol 35:254–260
Yang CH, Liu WH (2007) Cloning and characterization of a maltotriose-producing α-amylase gene from Thermobifida fusca. J Ind Microbiol Biotechnol 34:325–330
Zamost BL, Nielsen HK, Starnes RL (1991) Thermostable enzymes for industrial application. J Ind Microbiol 8:71–82
Cheng YF, Yang CH, Liu WH (2005) Cloning and expression of Thermobifida xylanase gene in the methylotrophic yeast Pichia pastoris. Enzyme Microb Technol 37:541–546
Yang CH, Huang YC, Chen CY, Wen CY (2010) Expression of Thermobifida fusca thermostable raw starch digesting alpha-amylase in Pichia pastoris and its application in raw sago starch hydrolysis. J Ind Microbiol Biotechnol. doi:10.1007/s10295-009-0686-9
Fickers P, Benetti PH, Wache Y, Marty A (2005) Hydrophobic substrate utilization by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543
Madzak C, Treton B, Blanchin-Roland S (2000) Strong hybrid promoters and integrative expression/secretion vectors for quasi-constitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. J Mol Microbiol Biotechnol 2:207–216
Madzak C, Gaillardin C, Beckerich JM (2004) Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica. J Biotechnol 109:63–81
Barth G, Gaillardin C (1997) Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev 19:219–237
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:533–550
Mortensen PB, Clausen MR (1996) Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol 31:32–148
Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A (1994) Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr 60:544–551
Philip J, Muir JG, Birkett A, Lu ZX, Jones GP, O’Dea K, Young GP (1995) Effect of resistant starch on fecal bulk and fermentation-dependent events in humans. Am J Clin Nutr 62:121–130
Lin JH, Wang SW, Chang YH (2009) Impacts of acid-methanol treatment and annealing on the enzymatic resistance of corn starches. Food Hydrocoll 23:1465–1472
Eerlingen RC, Deceuninck M, Delcour JA (1993) Enzyme-resistant starch. II. Influence of amylose chain length on resistant starch formation. Cereal Chem 70:345–350
Leong YH, Karim AA, Norziah MH (2007) Effect of pullulanase debranching of sago (Metroxylon sagu) starch at subgelatinization temperature on the yield of resistant starch. Starch 59:21–32
Brumovsky JO, Thompson DB (2001) Production of boiling-stable granular resistant starch by partial acid hydrolysis and hydrothermal treatment of high-amylose maize starch. Cereal Chem 78:680–689
Xuan JW, Fournier P, Gaillardin C (1988) Cloning of the LYS5 gene encoding saccharopine dehydrogenase from the yeast Yarrowia lipolytica by target integration. Curr Genet 14:15–21
Lin JH, Lee SY, Chang YH (2003) Effect of acid-alcohol treatment on the molecular structure and physiochemical properties of maize and potato starches. Carbohydr Polym 53:475–482
Lin JH, Chang YH, Hsu YH (2009) Degradation of cotton cellulose treated with hydrochloric acid either in water or in ethanol. Food Hydrocoll 23:1548–1553
Bordes F, Fudalej F, Dossat V, Nicaud JM, Marty A (2007) A new recombinant protein expression system for high-throughput screening in the yeast Yarrowia lipolytica. J Microbiol Methods 70:393–502
Madzak C, Otterbein L, Chamkha M, Moukha S, Asther M, Gaillardin C, Beckerich JM (2005) Heterologous production of a laccase from the basidiomycete Pycnoporus cinnabarinus in the dimorphic yeast Yarrowia lipolytica. FEMS Yeast Res 5:635–646
Haralampu SG (2000) Resistant starch—a review of the physical properties and biological impact of RS3. Carbohydr Polym 41:285–292
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, CH., Huang, YC., Chen, CY. et al. Heterologous expression of Thermobifida fusca thermostable alpha-amylase in Yarrowia lipolytica and its application in boiling stable resistant sago starch preparation. J Ind Microbiol Biotechnol 37, 953–960 (2010). https://doi.org/10.1007/s10295-010-0745-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0745-2